Nrf2/antioxidant pathway mediates β cell self-repair after damage by high-fat diet-induced oxidative stress
- PMID: 29263299
- PMCID: PMC5752274
- DOI: 10.1172/jci.insight.92854
Nrf2/antioxidant pathway mediates β cell self-repair after damage by high-fat diet-induced oxidative stress
Abstract
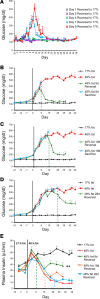
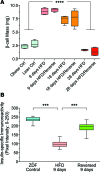
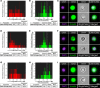
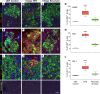
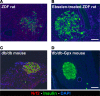
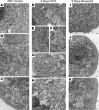
Many theories have been advanced to better understand why β cell function and structure relentlessly deteriorate during the course of type 2 diabetes (T2D). These theories include inflammation, apoptosis, replication, neogenesis, autophagy, differentiation, dedifferentiation, and decreased levels of insulin gene regulatory proteins. However, none of these have considered the possibility that endogenous self-repair of existing β cells may be an important factor. To examine this hypothesis, we conducted studies with female Zucker diabetic fatty rats fed a high-fat diet (HFD) for 1, 2, 4, 7, 9, 18, or 28 days, followed by a return to regular chow for 2-3 weeks. Repair was defined as reversal of elevated blood glucose and of inappropriately low blood insulin levels caused by a HFD, as well as reversal of structural damage visualized by imaging studies. We observed evidence of functional β cell damage after a 9-day exposure to a HFD and then repair after 2-3 weeks of being returned to normal chow (blood glucose [BG] = 348 ± 30 vs. 126 ± 3; mg/dl; days 9 vs. 23 day, P < 0.01). After 18- and 28-day exposure to a HFD, damage was more severe and repair was less evident. Insulin levels progressively diminished with 9-day exposure to a HFD; after returning to a regular diet, insulin levels rebounded toward, but did not reach, normal values. Increase in β cell mass was 4-fold after 9 days and 3-fold after 18 days, and there was no increase after 28 days of a HFD. Increases in β cell mass during a HFD were not different when comparing values before and after a return to regular diet within the 9-, 18-, or 28-day studies. No changes were observed in apoptosis or β cell replication. Formation of intracellular markers of oxidative stress, intranuclear translocation of Nrf2, and formation of intracellular antioxidant proteins indicated the participation of HFD/oxidative stress induction of the Nrf2/antioxidant pathway. Flow cytometry-based assessment of β cell volume, morphology, and insulin-specific immunoreactivity, as well as ultrastructural analysis by transmission electron microscopy, revealed that short-term exposure to a HFD produced significant changes in β cell morphology and function that are reversible after returning to regular chow. These results suggest that a possible mechanism mediating the ability of β cells to self-repair after a short-term exposure to a HFD is the activation of the Nrf2/antioxidant pathway.
Keywords: Beta cells; Cell stress; Insulin; Metabolism.
Conflict of interest statement
Figures

References
-
- Santin I, Eizirik DL. Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and β-cell apoptosis. Diabetes Obes Metab. 2013;15 Suppl 3:71–81. - PubMed
-
- Donath MY. Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obes Metab. 2013;15 Suppl 3:193–196. - PubMed
-
- Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 Suppl 2:S97–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

