PCH1 and PCHL promote photomorphogenesis in plants by controlling phytochrome B dark reversion
- PMID: 29263319
- PMCID: PMC5738371
- DOI: 10.1038/s41467-017-02311-8
PCH1 and PCHL promote photomorphogenesis in plants by controlling phytochrome B dark reversion
Abstract
Phytochrome B (phyB) is the primary red light photoreceptor in plants, and regulates both growth and development. The relative levels of phyB in the active state are determined by the light conditions, such as direct sunlight or shade, but are also affected by light-independent dark reversion. Dark reversion is a temperature-dependent thermal relaxation process, by which phyB reverts from the active to the inactive state. Here, we show that the homologous phyB-binding proteins PCH1 and PCHL suppress phyB dark reversion, resulting in plants with dramatically enhanced light sensitivity. Moreover, far-red and blue light upregulate the expression of PCH1 and PCHL in a phyB independent manner, thereby increasing the response to red light perceived by phyB. PCH1 and PCHL therefore provide a node for the molecular integration of different light qualities by regulation of phyB dark reversion, allowing plants to adapt growth and development to the ambient environment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

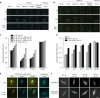

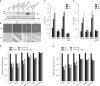
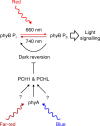
References
-
- Mancinelli, A. L. in Photomorphogenesis inPlants (eds Kendrick, R. E. & Kronenberg, G. H. M.) 211–269 (Kluwer Academic Publishers, Dordrecht, 1994).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

