Evaluation of serum MMP-9 as predictive biomarker for antisense therapy in Duchenne
- PMID: 29263366
- PMCID: PMC5738430
- DOI: 10.1038/s41598-017-17982-y
Evaluation of serum MMP-9 as predictive biomarker for antisense therapy in Duchenne
Abstract
Duchenne Muscular Dystrophy (DMD) is a severe muscle disorder caused by lack of dystrophin. Predictive biomarkers able to anticipate response to the therapeutic treatments aiming at dystrophin re-expression are lacking. The objective of this study is to investigate Matrix Metalloproteinase-9 (MMP-9) as predictive biomarker for Duchenne. Two natural history cohorts were studied including 168 longitudinal samples belonging to 66 patients. We further studied 1536 samples obtained from 3 independent clinical trials with drisapersen, an antisense oligonucleotide targeting exon 51: an open label study including 12 patients; a phase 3 randomized, double blind, placebo controlled study involving 186 patients; an open label extension study performed after the phase 3. Analysis of natural history cohorts showed elevated MMP-9 levels in patients and a significant increase over time in longitudinal samples. MMP-9 decreased in parallel to clinical stabilization in the 12 patients involved in the open label study. The phase 3 study and subsequent extension study clarified that the decrease in MMP-9 levels was not predictive of treatment response. These data do not support the inclusion of serum MMP-9 as predictive biomarker for DMD patients.
Conflict of interest statement
LUMC has patents on exon skipping of some of which A.A.R. and P.A.C.t.H. are co-inventors. Upon sublicensing some of these patents to Biomarin, A.A.R. and P.A.C.t.H. have received a share of royalty payments from LUMC. J.J.G.M.V. and E.H.N. report to be involved in clinical trials for Duchenne muscular dystrophy for GSK, Biomarin and Santhera. LUMC receives research funding from the Association française contre les myopathies, the EU, ZonMW, NOW, Prinses Beatrix Spierfonds, PPMD and DPP-NL. A.L., K.K., R.J.B., G.C. and S.d.K. report being employed by Biomarin. F.M. reports having served on scientific advisory boards for Acceleron Pharma, Genzyme, AVI BioPharma, Debiopharma Group, GSK, Biomarin, Servier and Santhera Pharmaceutical. F.M. serves on the editorial board of Neuromuscular Disorders and Neuropediatrics; receives research support from the European Union, the MRC, the Wellcome Trust, the Association Française Contre les Myopathies (AFM), the Muscular Dystrophy Campaign, the GOSH Biomedical Research Centre and the Muscular Dystrophy Association USA, is receiving funding for trials from GSK and the British Heart Foundation and has received funding for trials from AVI BioPharma, Trophos and PTC.
Figures
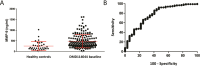




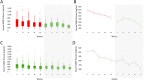
References
-
- Henricson EK, et al. Thecooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and othe. Muscle Nerve. 2013;48:55–67. doi: 10.1002/mus.23808. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

