Deciphering the emergence, genetic diversity and evolution of classical swine fever virus
- PMID: 29263428
- PMCID: PMC5738429
- DOI: 10.1038/s41598-017-18196-y
Deciphering the emergence, genetic diversity and evolution of classical swine fever virus
Abstract
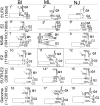
Classical swine fever (CSF) is one of the most important infectious diseases causing significant economic losses. Its causal agent, CSF virus (CSFV), is a member of the Pestivirus genus included into the Flaviviridae family. Previous molecular epidemiology studies have revealed the CSFV diversity is divided into three main genotypes and different subgenotypes. However, the classification system for CSFV has not yet been harmonized internationally. Similarly, the phylogeny and evolutionary dynamics of CSFV remain unclear. The current study provides novel and significant insights into the origin, diversification and evolutionary process of CSFV. In addition, the best phylogenetic marker for CSFV capable of reproducing the same phylogenetic and evolutionary information as the complete viral genome is characterized. Also, a reliable cut-off to accurately classify CSFV at genotype and subgenotype levels is established. Based on the time for the most recent common ancestor (tMRCA) reconstruction and cophylogenetic analysis, it was determined that CSFV emerged around 225 years ago when the Tunisian Sheep Virus jumped from its natural host to swine. CSFV emergence was followed by a genetic expansion in three main lineages, driven by the action of positive selection pressure and functional divergence, as main natural forces.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

