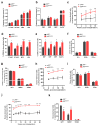
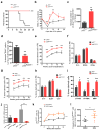
Genotoxic stresses promote clonal expansion of hematopoietic stem cells expressing mutant p53
- PMID: 29263439
- PMCID: PMC5842141
- DOI: 10.1038/leu.2017.325
Genotoxic stresses promote clonal expansion of hematopoietic stem cells expressing mutant p53
Conflict of interest statement
The authors declared that no conflict interest exists.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

