Long-term outcomes in half-dose verteporfin photodynamic therapy for chronic central serous retinopathy
- PMID: 29263642
- PMCID: PMC5724425
- DOI: 10.2147/OPTH.S151933
Long-term outcomes in half-dose verteporfin photodynamic therapy for chronic central serous retinopathy
Abstract
Objective: To evaluate the short- and long-term outcomes of half-dose verteporfin with photodynamic therapy (PDT) in the treatment of chronic central serous retinopathy (CSR).
Design: Retrospective case series.
Participants: 45 eyes in 39 patients with chronic CSR were included. Diagnosis of chronic CSR was confirmed by fluorescein angiography and persistence of subretinal fluid by optical coherence tomography for a minimum of 3 months duration.
Methods: Each patient underwent treatment with half-dose verteporfin with full-fluence PDT; initial follow-up was defined as a 6-8 week visit following the treatment, and final follow-up ranged from 5 to 70 months.
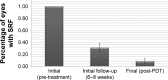
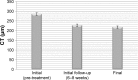
Results: The average follow-up period for treatment was 19.3 months. Best-corrected visual acuity increased from logMAR means of 0.52 to 0.42 (p<0.05). Central retinal thickness and choroidal thickness also significantly decreased at last follow-up (p<0.05). Eight of 45 eyes (18%) demonstrated a recurrence of CSR following treatment within the follow-up period. At the final follow-up, 41 out of the 45 eyes (91%) had complete resolution of subretinal fluid accumulation.
Conclusion: Half-dose PDT is an effective treatment option for chronic CSR in a Canadian population, and it is both safe and durable. The positive treatment effect is realized rapidly, with the initial 6-week result highly correlated with the final follow-up result.
Keywords: central serous chorioretinopathy; half-dose verteporfin; photodynamic therapy; verteporfin.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Piccolino FC, Borgia L, Zinicola E, Zingirian M. Indocyanine green angiographic findings in central serous chorioretinopathy. Eye (Lond) 1995;9(Pt 3):324–332. - PubMed
-
- Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41(2):201–214. - PubMed
-
- Wang M, Munch IC, Hasler PW, Prunte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–145. - PubMed
-
- Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials – TAP report. treatment of age-related macular degeneration with photodynamic therapy (TAP) study group. Arch Ophthalmol. 1999;117(10):1329–1345. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

