Selective arc-discharge synthesis of Dy2S-clusterfullerenes and their isomer-dependent single molecule magnetism
- PMID: 29263779
- PMCID: PMC5734629
- DOI: 10.1039/C7SC02395B
Selective arc-discharge synthesis of Dy2S-clusterfullerenes and their isomer-dependent single molecule magnetism
Abstract
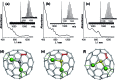
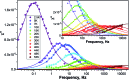
A method for the selective synthesis of sulfide clusterfullerenes Dy2S@C2n is developed. Addition of methane to the reactive atmosphere reduces the formation of empty fullerenes in the arc-discharge synthesis, whereas the use of Dy2S3 as a source of metal and sulfur affords sulfide clusterfullerenes as the main fullerene products along with smaller amounts of carbide clusterfullerenes. Two isomers of Dy2S@C82 with Cs(6) and C3v(8) cage symmetry, Dy2S@C72-Cs(10528), and a carbide clusterfullerene Dy2C2@C82-Cs(6) were isolated. The molecular structure of both Dy2S@C82 isomers was elucidated by single-crystal X-ray diffraction. SQUID magnetometry demonstrates that all of these clusterfullerenes exhibit hysteresis of magnetization, with Dy2S@C82-C3v(8) being the strongest single molecule magnet in the series. DC- and AC-susceptibility measurements were used to determine magnetization relaxation times in the temperature range from 1.6 K to 70 K. Unprecedented magnetization relaxation dynamics with three consequent Orbach processes and energy barriers of 10.5, 48, and 1232 K are determined for Dy2S@C82-C3v(8). Dy2S@C82-Cs(6) exhibits faster relaxation of magnetization with two barriers of 15.2 and 523 K. Ab initio calculations were used to interpret experimental data and compare the Dy-sulfide clusterfullerenes to other Dy-clusterfullerenes. The smallest and largest barriers are ascribed to the exchange/dipolar barrier and relaxation via crystal-field states, respectively, whereas an intermediate energy barrier of 48 K in Dy2S@C82-C3v(8) is assigned to the local phonon mode, corresponding to the librational motion of the Dy2S cluster inside the carbon cage.
Figures








References
-
- Sessoli R., Gatteschi D., Caneschi A., Novak M. A. Nature. 1993;365:141–143.
-
- Ishikawa N., Sugita M., Ishikawa T., Koshihara S., Kaizu Y. J. Am. Chem. Soc. 2003;125:8694–8695. - PubMed
-
- Feltham H. L. C., Brooker S. Coord. Chem. Rev. 2014;276:1–33.
-
- Layfield R. A. Organometallics. 2014;33:1084–1099.
-
- Habib F., Murugesu M. Chem. Soc. Rev. 2013;42:3278–3288. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

