Familial STAG2 germline mutation defines a new human cohesinopathy
- PMID: 29263825
- PMCID: PMC5677968
- DOI: 10.1038/s41525-017-0009-4
Familial STAG2 germline mutation defines a new human cohesinopathy
Abstract
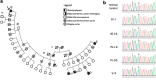
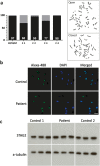
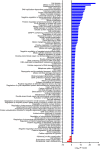
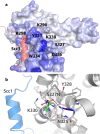
We characterize a novel human cohesinopathy originated from a familial germline mutation of the gene encoding the cohesin subunit STAG2, which we propose to call STAG2-related X-linked Intellectual Deficiency. Five individuals carry a STAG2 p.Ser327Asn (c.980 G > A) variant that perfectly cosegregates with a phenotype of syndromic mental retardation in a characteristic X-linked recessive pattern. Although patient-derived cells did not show overt sister-chromatid cohesion defects, they exhibited altered cell cycle profiles and gene expression patterns that were consistent with cohesin deficiency. The protein level of STAG2 in patient cells was normal. Interestingly, STAG2 S327 is located at a conserved site crucial for binding to SCC1 and cohesin regulators. When expressed in human cells, the STAG2 p.Ser327Asn mutant is defective in binding to SCC1 and other cohesin subunits and regulators. Thus, decreased amount of intact cohesin likely underlies the phenotypes of STAG2-SXLID. Intriguingly, recombinant STAG2 p.Ser327Asn binds normally to SCC1, WAPL, and SGO1 in vitro, suggesting the existence of unknown in vivo mechanisms that regulate the interaction between STAG2 and SCC1.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

