Workflow optimization of whole genome amplification and targeted panel sequencing for CTC mutation detection
- PMID: 29263843
- PMCID: PMC5677973
- DOI: 10.1038/s41525-017-0034-3
Workflow optimization of whole genome amplification and targeted panel sequencing for CTC mutation detection
Abstract
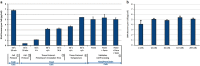
Genomic characterization of circulating tumor cells (CTCs) may prove useful as a surrogate for conventional tissue biopsies. This is particularly important as studies have shown different mutational profiles between CTCs and ctDNA in some tumor subtypes. However, isolating rare CTCs from whole blood has significant hurdles. Very limited DNA quantities often can't meet NGS requirements without whole genome amplification (WGA). Moreover, white blood cells (WBC) germline contamination may confound CTC somatic mutation analyses. Thus, a good CTC enrichment platform with an efficient WGA and NGS workflow are needed. Here, Vortex label-free CTC enrichment platform was used to capture CTCs. DNA extraction was optimized, WGA evaluated and targeted NGS tested. We used metastatic colorectal cancer (CRC) as the clinical target, HCT116 as the corresponding cell line, GenomePlex® and REPLI-g as the WGA methods, GeneRead DNAseq Human CRC Panel as the 38 gene panel. The workflow was further validated on metastatic CRC patient samples, assaying both tumor and CTCs. WBCs from the same patients were included to eliminate germline contaminations. The described workflow performed well on samples with sufficient DNA, but showed bias for rare cells with limited DNA input. REPLI-g provided an unbiased amplification on fresh rare cells, enabling an accurate variant calling using the targeted NGS. Somatic variants were detected in patient CTCs and not found in age matched healthy donors. This demonstrates the feasibility of a simple workflow for clinically relevant monitoring of tumor genetics in real time and over the course of a patient's therapy using CTCs.
Conflict of interest statement
Some of the authors (H.E.L., M.V., J.C., C.R., E.S.C.) have financial interests in Vortex Biosciences and intellectual property described herein. All the other authors declare that they have no competing financial interests.
Figures






References
-
- Pantel, K. & Speicher, M.R. The biology of circulating tumor cells. Oncogene10.1038/onc.2015.192 (2015). - PubMed
-
- Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650–661. doi: 10.1158/2159-8290.CD-13-1014. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

