Enhancing vaccine antibody responses by targeting Clec9A on dendritic cells
- PMID: 29263886
- PMCID: PMC5674066
- DOI: 10.1038/s41541-017-0033-5
Enhancing vaccine antibody responses by targeting Clec9A on dendritic cells
Abstract
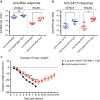
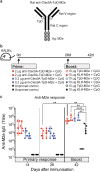
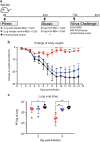
Targeting model antigens (Ags) to Clec9A on DC has been shown to induce, not only cytotoxic T cells, but also high levels of Ab. In fact, Ab responses against immunogenic Ag were effectively generated even in the absence of DC-activating adjuvants. Here we tested if targeting weakly immunogenic putative subunit vaccine Ags to Clec9A could enhance Ab responses to a level likely to be protective. The proposed "universal" influenza Ag, M2e and the enterovirus 71 Ag, SP70 were linked to anti-Clec9A Abs and injected into mice. Targeting these Ags to Clec9A greatly increased Ab titres. For optimal responses, a DC-activating adjuvant was required. For optimal responses, a boost injection was also needed, but the high Ab titres against the targeting construct blocked Clec9A-targeted boosting. Heterologous prime-boost strategies avoiding cross-reactivity between the priming and boosting targeting constructs overcame this limitation. In addition, targeting small amounts of Ag to Clec9A served as an efficient priming for a conventional boost with higher levels of untargeted Ag. Using this Clec9A-targeted priming, conventional boosting strategy, M2e immunisation protected mice from infection with lethal doses of influenza H1N1 virus.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



Similar articles
-
Targeting Antigen to Clec9A Primes Follicular Th Cell Memory Responses Capable of Robust Recall.J Immunol. 2015 Aug 1;195(3):1006-14. doi: 10.4049/jimmunol.1500767. Epub 2015 Jun 22. J Immunol. 2015. PMID: 26101322
-
Evolution of B cell responses to Clec9A-targeted antigen.J Immunol. 2013 Nov 15;191(10):4919-25. doi: 10.4049/jimmunol.1301947. Epub 2013 Oct 11. J Immunol. 2013. PMID: 24123689
-
Antibody-mediated targeting of antigen to C-type lectin-like receptors Clec9A and Clec12A elicits different vaccination outcomes.Mol Immunol. 2017 Jan;81:143-150. doi: 10.1016/j.molimm.2016.12.010. Epub 2016 Dec 12. Mol Immunol. 2017. PMID: 27978488
-
Boosting antibody responses by targeting antigens to dendritic cells.Trends Immunol. 2012 Feb;33(2):71-7. doi: 10.1016/j.it.2011.10.007. Epub 2011 Dec 6. Trends Immunol. 2012. PMID: 22153931 Review.
-
Exploring Clec9a in dendritic cell-based tumor immunotherapy for molecular insights and therapeutic potentials.NPJ Vaccines. 2025 Feb 7;10(1):27. doi: 10.1038/s41541-025-01084-2. NPJ Vaccines. 2025. PMID: 39920156 Free PMC article. Review.
Cited by
-
Biomaterials to enhance antigen-specific T cell expansion for cancer immunotherapy.Biomaterials. 2021 Jan;268:120584. doi: 10.1016/j.biomaterials.2020.120584. Epub 2020 Dec 5. Biomaterials. 2021. PMID: 33338931 Free PMC article. Review.
-
Selectively targeting haemagglutinin antigen to chicken CD83 receptor induces faster and stronger immunity against avian influenza.NPJ Vaccines. 2021 Jul 15;6(1):90. doi: 10.1038/s41541-021-00350-3. NPJ Vaccines. 2021. PMID: 34267228 Free PMC article.
-
A DNA Vaccine Encoding the Gn Ectodomain of Rift Valley Fever Virus Protects Mice via a Humoral Response Decreased by DEC205 Targeting.Front Immunol. 2019 Apr 25;10:860. doi: 10.3389/fimmu.2019.00860. eCollection 2019. Front Immunol. 2019. PMID: 31105695 Free PMC article.
-
Autotransporter-Mediated Display of Complement Receptor Ligands by Gram-Negative Bacteria Increases Antibody Responses and Limits Disease Severity.Pathogens. 2020 May 14;9(5):375. doi: 10.3390/pathogens9050375. Pathogens. 2020. PMID: 32422907 Free PMC article.
-
Specific targeting of IL-1β activity to CD8+ T cells allows for safe use as a vaccine adjuvant.NPJ Vaccines. 2020 Jul 23;5(1):64. doi: 10.1038/s41541-020-00211-5. eCollection 2020. NPJ Vaccines. 2020. PMID: 32714571 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

