Developing a platform system for gene delivery: amplifying virus-like particles (AVLP) as an influenza vaccine
- PMID: 29263887
- PMCID: PMC5696535
- DOI: 10.1038/s41541-017-0031-7
Developing a platform system for gene delivery: amplifying virus-like particles (AVLP) as an influenza vaccine
Abstract
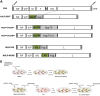
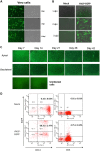
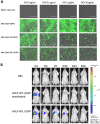
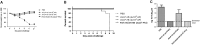
Delivery of a gene of interest to target cells is highly desirable for translational medicine, such as gene therapy, regenerative medicine, vaccine development, and studies of gene function. Parainfluenza virus 5 (PIV5), a paramyxovirus with a negative-sense RNA genome, normally infects cells without causing obvious cytopathic effect, and it can infect many cell types. To exploit these features of PIV5, we established a system generating self-amplifying, virus-like particles (AVLP). Using enhanced green fluorescent protein (EGFP) as a reporter, AVLP encoding EGFP (AVLP-EGFP) successfully delivered and expressed the EGFP gene in primary human cells, including stem cells, airway epithelial cells, monocytes, and T cells. To demonstrate the application of this system for vaccine development, we generated AVLPs to express the HA and M1 antigens from the influenza A virus strain H5N1 (AVLP-H5 and AVLP-M1H5). Immunization of mice with AVLP-H5 and AVLP-M1H5 generated robust antibody and cellular immune responses. Vaccination with a single dose of AVLP-H5 and M1H5 completely protected mice against lethal H5N1 challenge, suggesting that the AVLP-based system is a promising platform for delivery of desirable genes.
Conflict of interest statement
B.H. is the inventor of patent application “PIV5-based AVLP” that is being pursued by the University of Georgia Research Foundation. The remaining authors declare no competing financial interests.
Figures

References
-
- Lamb, R. A. & Kolakofsky, D. in Fields Virology 4th edn. (eds Knipe D. M. & Howley P. M.) (Lippincott, Williams and Wilkins, 2001).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

