Prohibitin: a potential therapeutic target in tyrosine kinase signaling
- PMID: 29263933
- PMCID: PMC5730683
- DOI: 10.1038/sigtrans.2017.59
Prohibitin: a potential therapeutic target in tyrosine kinase signaling
Abstract
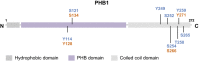
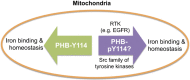
Prohibitin is a pleiotropic protein that has roles in fundamental cellular processes, such as cellular proliferation and mitochondrial housekeeping, and in cell- or tissue-specific functions, such as adipogenesis and immune cell functions. The different functions of prohibitin are mediated by its cell compartment-specific attributes, which include acting as an adaptor molecule in membrane signaling, a scaffolding protein in mitochondria, and a transcriptional co-regulator in the nucleus. However, the precise relationship between its distinct cellular localization and diverse functions remain largely unknown. Accumulating evidence suggests that the phosphorylation of prohibitin plays a role in a number of cell signaling pathways and in intracellular trafficking. Herein, we discuss the known and potential importance of the site-specific phosphorylation of prohibitin in regulating these features. We will discuss this in the context of new evidence from tissue-specific transgenic mouse models of prohibitin, including a mutant prohibitin lacking a crucial tyrosine phosphorylation site. We conclude with the opinion that prohibitin can be used as a potential target for tyrosine kinase signal transduction-targeting therapy, including in insulin, growth factors, and immune signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- McClung JK, Danner DB, Stewart DA, Smith JR, Schneider EL, Lumpkin CK et al. Isolation of a cDNA that hybrid selects antiproliferative mRNA from rat liver. Biochem Biophys Res Commun 1989; 164: 1316–1322. - PubMed
-
- White JJ, Ledbetter DH, Eddy Jr RL, Shows TB, Stewart DA, Nuell MJ et al. Assignment of the human prohibitin gene (PHB1) to chromosome 17 and identification of a DNA polymorphism. Genomics 1991; 11: 228–230. - PubMed
-
- McClung JK, King RL, Walker LS, Danner DB, Nuell MJ, Stewart CA et al. Expression of prohibitin, an antiproliferative protein. Exp Gerontol 1992; 27: 413–417. - PubMed
-
- Manjeshwar S, Branam DE, Lerner MR, Brackett DJ, Jupe ER. Tumor suppression by the prohibitin gene 3'untranslated region RNA in human breast cancer. Cancer Res 2003; 63: 5251–5256. - PubMed
-
- Nadimpalli R, Yalpani N, Johal GS, Simmons CR. Prohibitins, stomatins, and plant disease response genes compose a protein superfamily that controls cell proliferation, ion channel regulation, and death. J Biol Chem 2000; 275: 29579–29586. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

