Corticotropinoma as a Component of Carney Complex
- PMID: 29264542
- PMCID: PMC5686778
- DOI: 10.1210/js.2017-00231
Corticotropinoma as a Component of Carney Complex
Abstract
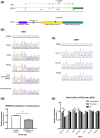
Known germline gene abnormalities cause one-fifth of the pituitary adenomas in children and adolescents, but, in contrast with other pituitary tumor types, the genetic causes of corticotropinomas are largely unknown. In this study, we report a case of Cushing disease (CD) due to a loss-of-function mutation in PRKAR1A, providing evidence for association of this gene with a corticotropinoma. A 15-year-old male presenting with hypercortisolemia was diagnosed with CD. Remission was achieved after surgical resection of a corticotropin (ACTH)-producing pituitary microadenoma, but recurrence 3 years later prompted reoperation and radiotherapy. Five years after the original diagnosis, the patient developed ACTH-independent Cushing syndrome, and a diagnosis of primary pigmented nodular adrenocortical disease was confirmed. A PRKAR1A mutation (c.671delG, p.G225Afs*16) was detected in a germline DNA sample from the patient, which displayed loss of heterozygosity in the corticotropinoma. No other germline or somatic mutations of interest were found. As corticotropinomas are not a known component of Carney complex (CNC), we performed loss of heterozygosity and messenger RNA stability studies in the patient's tissues, and analyzed the effect of Prkar1a silencing on AtT-20/D16v-F2 mouse corticotropinoma cells. No PRKAR1A defects were found among 97 other pediatric CD patients studied. Our clinical case and experimental data support a role for PRKAR1A in the pathogenesis of a corticotroph cell tumor. This is a molecularly confirmed report of a corticotropinoma presenting in association with CNC. We conclude that germline PRKAR1A mutations are a novel, albeit apparently infrequent, cause of CD.
Keywords: Carney complex; Cushing disease; genetics; pituitary tumor; protein kinase A.
Figures

References
-
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26(1):89–92. - PubMed
-
- Armstrong DK, Irvine AD, Handley JM, Walsh MY, Hadden DR, Bingham EA. Carney complex: report of a kindred with predominantly cutaneous manifestations. Br J Dermatol. 1997;136(4):578–582. - PubMed
-
- Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94(6):2085–2091. - PMC - PubMed
-
- Stratakis CA, Tichomirowa MA, Boikos S, Azevedo MF, Lodish M, Martari M, Verma S, Daly AF, Raygada M, Keil MF, Papademetriou J, Drori-Herishanu L, Horvath A, Tsang KM, Nesterova M, Franklin S, Vanbellinghen JF, Bours V, Salvatori R, Beckers A. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. 2010;78(5):457–463. - PMC - PubMed
-
- Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore). 1985;64(4):270–283. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
