Oxidative stress triggers aggregation of GFP-tagged Hsp31p, the budding yeast environmental stress response chaperone, and glyoxalase III
- PMID: 29264711
- PMCID: PMC6045530
- DOI: 10.1007/s12192-017-0868-8
Oxidative stress triggers aggregation of GFP-tagged Hsp31p, the budding yeast environmental stress response chaperone, and glyoxalase III
Abstract
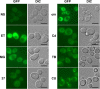
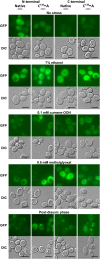
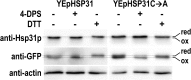
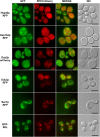
The Saccharomyces cerevisiae Hsp31p protein belongs to the ubiquitous DJ-1/ThiJ/PfpI family. The most prominent member of this family is human DJ-1; defects of this protein are associated with Parkinson's disease pathogenesis. Numerous recent findings reported by our group and others have revealed the importance of Hsp31p for survival in the post-diauxic phase of cell growth and under diverse environmental stresses. Hsp31p was shown to possess glutathione-independent glyoxalase III activity and to function as a protein chaperone, suggesting that it has multiple cellular roles. Our previous work also revealed that HSP31 gene expression was controlled by multiple stress-related transcription factors, which mediated HSP31 promoter responses to oxidative, osmotic, and thermal stresses, toxic products of glycolysis, and the diauxic shift. Nevertheless, the exact role of Hsp31p within budding yeast cells remains elusive. Here, we aimed to obtain insights into the function of Hsp31p based on its intracellular localization. We have demonstrated that the Hsp31p-GFP fusion protein is localized to the cytosol under most environmental conditions and that it becomes particulate in response to oxidative stress. However, the particles do not colocalize with other granular subcellular structures present in budding yeast cells. The observed particulate localization does not seem to be important for Hsp31p functionality. Instead, it is likely the result of oxidative damage, as the particle abundance increases when Hsp31p is nonfunctional, when the cellular oxidative stress response is affected, or when cellular maintenance systems that optimize the state of the proteome are compromised.
Keywords: Environmental stresses; Protein aggregates; Protein stability; Saccharomyces cerevisiae.
Figures


Similar articles
-
The budding yeast orthologue of Parkinson's disease-associated DJ-1 is a multi-stress response protein protecting cells against toxic glycolytic products.Biochim Biophys Acta Mol Cell Res. 2017 Jan;1864(1):39-50. doi: 10.1016/j.bbamcr.2016.10.016. Epub 2016 Oct 27. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27984092
-
Robust glyoxalase activity of Hsp31, a ThiJ/DJ-1/PfpI family member protein, is critical for oxidative stress resistance in Saccharomyces cerevisiae.J Biol Chem. 2015 Oct 30;290(44):26491-507. doi: 10.1074/jbc.M115.673624. Epub 2015 Sep 14. J Biol Chem. 2015. PMID: 26370081 Free PMC article.
-
Hsp31, a member of the DJ-1 superfamily, is a multitasking stress responder with chaperone activity.Prion. 2016 Mar 3;10(2):103-11. doi: 10.1080/19336896.2016.1141858. Prion. 2016. PMID: 27097320 Free PMC article.
-
Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system.Microbiol Mol Biol Rev. 2012 Jun;76(2):115-58. doi: 10.1128/MMBR.05018-11. Microbiol Mol Biol Rev. 2012. PMID: 22688810 Free PMC article. Review.
-
Chaperoning prions: the cellular machinery for propagating an infectious protein?Bioessays. 2005 Aug;27(8):823-32. doi: 10.1002/bies.20267. Bioessays. 2005. PMID: 16015602 Review.
Cited by
-
Comparative Proteomics of Two Flor Yeasts in Sparkling Wine Fermentation: First Approach.Foods. 2025 Jan 16;14(2):282. doi: 10.3390/foods14020282. Foods. 2025. PMID: 39856948 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

