L-Acetyl-carnitine in Patients with Carpal Tunnel Syndrome: Effects on Nerve Protection, Hand Function and Pain
- PMID: 29264721
- PMCID: PMC5747578
- DOI: 10.1007/s40263-017-0476-2
L-Acetyl-carnitine in Patients with Carpal Tunnel Syndrome: Effects on Nerve Protection, Hand Function and Pain
Erratum in
-
Author Correction to: L-Acetyl-carnitine in Patients with Carpal Tunnel Syndrome: Effects on Nerve Protection, Hand Function and Pain.CNS Drugs. 2018 Mar;32(3):303. doi: 10.1007/s40263-018-0493-9. CNS Drugs. 2018. PMID: 29441455 Free PMC article.
Abstract
Background and aim: L-Acetyl-carnitine (LAC) exerts an energetic effect on nerves and muscles. Recently, preclinical experiments have demonstrated a central anti-nociceptive action.
Objective: Our objective was to assess the effects of LAC on neuroprotection, pain, and function in carpal tunnel syndrome (CTS), a very frequent chronic compressive neuropathy.
Methods: In a multicentre, examiner-blinded, clinical and neurophysiological 4-month study, we enrolled 82 patients and examined 120 hands with CTS of mild to moderate severity. Patients were assessed at baseline and 10, 60 and 120 days after treatment with LAC 500 mg twice daily (BID). All patients underwent a conduction study of the median nerve, the Boston Carpal Tunnel Questionnaire (BCTQ) and the Neuropathic Pain Symptom Inventory (NPSI). The primary endpoint was the sensory conduction velocity (SCV) of the median nerve.
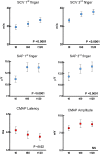
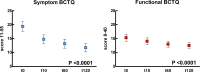
Results: The primary endpoint was met, with significant improvement of the SCV (P < 0.0001). All sensory neurophysiological measures also significantly improved. BCTQ score changed significantly (P < 0.0001), with a greater improvement in the symptom component. Nine of the NPSI types of pain, particularly squeezing and pressure pain and pain evoked by pressure, showed a significant reduction (P < 0.0001).
Conclusions: Our clinical and neurophysiological study indicated that 4 months of treatment with LAC exerted a neuroprotective effect. LAC reduced pain in patients with mild and moderate CTS, a result that is possibly due to both its neuroprotective action and its central anti-nociceptive properties. Clinical Trials Registration code: EudraCT 2014-002289-62.
Conflict of interest statement
Funding
The trial was funded by Sigma-Tau of ALFASIGMA Group. Open access was funded by Sigma-Tau of ALFASIGMA Group.
Conflict of interest
Giorgio Cruccu has received a research grant, consulting fees and payments for lectures from Sigma-Tau of ALFASIGMA Group, and consulting fees from Angelini, Biogen, and Mundipharma. Giulia Di Stefano, Francesco Fattapposta, and Luca Padua have no conflicts to declare. Stefano Jann has received payments for lectures and consulting fees from Sigma-Tau of ALFASIGMA Group and Gruenenthal and a research grant from Grifols. Angelo Schenone has received payments for lectures from Sigma-Tau of ALFASIGMA Group, Mundipharma, Ecupharma, Kedrion, CSL Behering, and Epitech. Andrea Truini has received payments for lectures from Sigma-Tau of ALFASIGMA Group and consulting fees or payment for lectures from Angelini, Gruenenthal and Pfizer.
Ethics approval
The study protocol, patient information and informed consent forms were reviewed and approved by the Institutional Review Board at the participant hospitals.
Consent to participate
All participants provided written informed consent before taking part in study procedures. All procedures were in accordance with Good Clinical Practice and the principles of the Declaration of Helsinki.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

