Toxicology Evaluation of Drugs Administered via Uncommon Routes: Intranasal, Intraocular, Intrathecal/Intraspinal, and Intra-Articular
- PMID: 29264927
- PMCID: PMC5874330
- DOI: 10.1177/1091581817741840
Toxicology Evaluation of Drugs Administered via Uncommon Routes: Intranasal, Intraocular, Intrathecal/Intraspinal, and Intra-Articular
Abstract
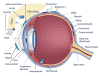
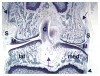
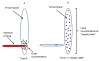
As the need for nasal, ocular, spinal, and articular therapeutic compounds increases, toxicology assessments of drugs administered via these routes play an important role in human safety. This symposium outlined the local and systemic evaluation to support safety during the development of these drugs in nonclinical models with some case studies. Discussions included selection of appropriate species for the intended route; conducting nonclinical studies that closely mimic the intended use with adequate duration; functional assessment, if deemed necessary; evaluation of local tissues with special histological staining procedure; and evaluations of safety margins based on local and systemic toxicity.
Keywords: analgesics; antispasticity; epidural; granuloma; intra-articular; intracameral; intranasal; intrathecal; intravitreal; local toxicity; safety evaluation; safety margins; spinal cord; toxicity.
Conflict of interest statement
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Illum L. Nasal clearance in health and disease. J Aerosol Med. 2006 Spring;19(1):92–9. - PubMed
-
- Grassin-Delyle S, Buenestado A, Naline E, Faisy C, Blouquit-Laye S, Couderc LJ, Le Guen M, Fischler M, Devillier P. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacol Ther. 2012 Jun;134(3):366–79. Reed (1993) - PubMed
-
- Fortuna A, Alves G, Serralheiro A, Sousa J, Falcão A. Intranasal delivery of systemic-acting drugs: small-molecules and biomacromolecules. Eur J Pharm Biopharm. 2014 Sep;88(1):8–27. - PubMed
-
- Mittal D, Ali A, Md S, Baboota S, Sahni JK, Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014 Mar;21(2):75–86. - PubMed
-
- Pires A, Fortuna A, Alves G, Falcão A. Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci. 2009;12(3):288–311. Review. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

