Affective and cooperative social interactions modulate effective connectivity within and between the mirror and mentalizing systems
- PMID: 29265483
- PMCID: PMC6866589
- DOI: 10.1002/hbm.23930
Affective and cooperative social interactions modulate effective connectivity within and between the mirror and mentalizing systems
Abstract
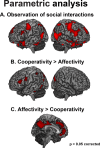
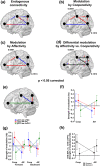
Decoding the meaning of others' actions, a crucial step for social cognition, involves different neural mechanisms. While the "mirror" and "mentalizing" systems have been associated with, respectively, the processing of biological actions versus more abstract information, their respective contribution to intention understanding is debated. Processing social interactions seems to recruit both neural systems, with a different weight depending on cues emphasizing either shared action goals or shared mental states. We have previously shown that observing cooperative and affective social interactions elicits stronger activity in key nodes of, respectively, the mirror (left posterior superior temporal sulcus (pSTS), superior parietal cortex (SPL), and ventral/dorsal premotor cortex (vPMC/dPMC)) and mentalizing (ventromedial prefrontal cortex (vmPFC)) systems. To unveil their causal organization, we investigated the effective connectivity underlying the observation of human social interactions expressing increasing cooperativity (involving left pSTS, SPL, and vPMC) versus affectivity (vmPFC) via dynamic causal modeling in 36 healthy human subjects. We found strong evidence for a model including the pSTS and vPMC as input nodes for the observed interactions. The extrinsic connectivity of this model undergoes oppositely valenced modulations, with cooperativity promoting positive modulations of connectivity between pSTS and both SPL (forward) and vPMC (mainly backward), and affectivity promoting reciprocal positive modulations of connectivity between pSTS and vmPFC (mainly backward). Alongside fMRI data, such divergent effective connectivity suggests that different dimensions underlying the processing of social interactions recruit distinct, although strongly interconnected, neural pathways associated with, respectively, the bottom-up visuomotor processing of motor intentions, and the top-down attribution of affective/mental states.
Keywords: dynamic causal modeling; effective connectivity; intention understanding; mentalizing system; mirror neuron system; social cognition; social interaction.
© 2017 Wiley Periodicals, Inc.
Figures

Similar articles
-
Increased pSTS activity and decreased pSTS-mPFC connectivity when processing negative social interactions.Behav Brain Res. 2021 Feb 5;399:113027. doi: 10.1016/j.bbr.2020.113027. Epub 2020 Nov 26. Behav Brain Res. 2021. PMID: 33249070
-
Action expectancy modulates activity in the mirror neuron system and mentalizing system.Neuroimage. 2024 Oct 15;300:120876. doi: 10.1016/j.neuroimage.2024.120876. Epub 2024 Sep 27. Neuroimage. 2024. PMID: 39343111
-
Reduced connectivity between mentalizing and mirror systems in autism spectrum condition.Neuropsychologia. 2019 Jan;122:88-97. doi: 10.1016/j.neuropsychologia.2018.11.008. Epub 2018 Nov 20. Neuropsychologia. 2019. PMID: 30468777
-
An integrative neural model of social perception, action observation, and theory of mind.Neurosci Biobehav Rev. 2015 Apr;51:263-75. doi: 10.1016/j.neubiorev.2015.01.020. Epub 2015 Feb 3. Neurosci Biobehav Rev. 2015. PMID: 25660957 Free PMC article. Review.
-
Understanding intentions from actions: Direct perception, inference, and the roles of mirror and mentalizing systems.Conscious Cogn. 2015 Nov;36:426-33. doi: 10.1016/j.concog.2015.03.012. Epub 2015 Apr 9. Conscious Cogn. 2015. PMID: 25864592 Review.
Cited by
-
Social Cognition through the Lens of Cognitive and Clinical Neuroscience.Biomed Res Int. 2018 Sep 13;2018:4283427. doi: 10.1155/2018/4283427. eCollection 2018. Biomed Res Int. 2018. PMID: 30302338 Free PMC article. Review.
-
The Precuneus Contributes to Embodied Scene Construction for Singing in an Opera.Front Hum Neurosci. 2021 Oct 15;15:737742. doi: 10.3389/fnhum.2021.737742. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34720910 Free PMC article.
-
Social Perception and Interaction Database-A Novel Tool to Study Social Cognitive Processes With Point-Light Displays.Front Psychiatry. 2020 Mar 11;11:123. doi: 10.3389/fpsyt.2020.00123. eCollection 2020. Front Psychiatry. 2020. PMID: 32218745 Free PMC article.
-
Neural representation of social concepts: a coordinate-based meta-analysis of fMRI studies.Brain Imaging Behav. 2021 Aug;15(4):1912-1921. doi: 10.1007/s11682-020-00384-6. Brain Imaging Behav. 2021. PMID: 32897484
-
The neurostructural bases of empathy: morphometric evidence for a multicomponential approach.Front Psychiatry. 2025 Apr 10;16:1544632. doi: 10.3389/fpsyt.2025.1544632. eCollection 2025. Front Psychiatry. 2025. PMID: 40276066 Free PMC article.
References
-
- Angelucci, A. , & Bressloff, P. C. (2006). Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra‐classical receptive field surround of primate V1 neurons. Progress in Brain Research, 154, 93–120. - PubMed
-
- Beauchamp, M. S. , Lee, K. E. , Haxby, J. V. , & Martin, A. (2002). Parallel visual motion processing streams for manipulable objects and human movements. Neuron, 34, 149–159. - PubMed
-
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57,
-
- Blakemore, S. J. , & Decety, J. (2001). From the perception of action to the understanding of intention. Nature Reviews. Neuroscience, 2, 561–567. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

