Five years of monitoring for the emergence of oseltamivir resistance in patients with influenza A infections in the Influenza Resistance Information Study
- PMID: 29265727
- PMCID: PMC5820429
- DOI: 10.1111/irv.12534
Five years of monitoring for the emergence of oseltamivir resistance in patients with influenza A infections in the Influenza Resistance Information Study
Abstract
Background and objectives: The Influenza Resistance Information Study (IRIS) was initiated in 2008 to study the emergence of neuraminidase inhibitor (NAI) resistance and the clinical course of influenza in immunocompetent treated and untreated patients.
Methods: Patients had throat/nose swabs collected on days 1, 3, 6 and 10 for analyses of influenza type, subtype and virus susceptibility to NAIs. RT-PCR-positive samples were cultured and tested for NAI resistance by specific RT-PCR and phenotypic testing. Scores for influenza symptoms were recorded on diary cards (Days 1-10). This study focuses on influenza A-infected cases only.
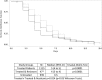
Results: Among 3230 RT-PCR-positive patients, 2316 had influenza A of whom 1216 received oseltamivir monotherapy within 2 days of symptom onset (9 seasonal H1N1; 662 H3N2; 545 H1N1pdm2009). Except for 9 patients with naturally resistant seasonal H1N1 (2008/9), no resistance was detected in Day 1 samples. Emergence of resistance (post-Day 1) was detected in 43/1207 (3.56%) oseltamivir-treated influenza A-infected patients, with a higher frequency in 1- to 5-year-olds (11.8%) vs >5-year-olds (1.4%). All N1- and N2-resistant viruses had H275Y (n = 27) or R292K (n = 16) substitutions, respectively. For 43 patients, virus clearance was significantly delayed vs treated patients with susceptible viruses (8.1 vs 10.9 days; P < .0001), and 11 (23.2%) remained RT-PCR positive for influenza at Day 10. However, their symptoms resolved by Day 6 or earlier.
Conclusions: Oseltamivir resistance was only detected during antiviral treatment, with the highest incidence occurring among 1- to 5-year-olds. Resistance delayed viral clearance, but had no impact on symptom resolution.
Keywords: antiviral; influenza; neuraminidase inhibitor; resistance.
© 2017 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Figures


References
-
- Hayden FG, Pavia AT. Antiviral management of seasonal and pandemic influenza. J Infect Dis. 2006;194:S119‐26. - PubMed
-
- Kim CU, Lew W, Williams MA, et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis and structural analysis of carbocyclic sialic acid analogues with potent anti‐influenza activity. J Am Chem Soc. 1997;119:681‐90. - PubMed
-
- Bright RA, Medina MJ, Xu X, et al. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366:1175‐81. - PubMed
-
- Aoki FY, Boivin G, Roberts N. Influenza virus susceptibility and resistance to oseltamivir. Antivir Ther. 2007;12:603‐16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

