Nemaline myopathy and distal arthrogryposis associated with an autosomal recessive TNNT3 splice variant
- PMID: 29266598
- PMCID: PMC5805634
- DOI: 10.1002/humu.23385
Nemaline myopathy and distal arthrogryposis associated with an autosomal recessive TNNT3 splice variant
Abstract
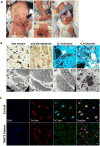
A male neonate presented with severe weakness, hypotonia, contractures and congenital scoliosis. Skeletal muscle specimens showed marked atrophy and degeneration of fast fibers with striking nemaline rods and hypertrophy of slow fibers that were ultrastructurally normal. A neuromuscular gene panel identified a homozygous essential splice variant in TNNT3 (chr11:1956150G > A, NM_006757.3:c.681+1G > A). TNNT3 encodes skeletal troponin-Tfast and is associated with autosomal dominant distal arthrogryposis. TNNT3 has not previously been associated with nemaline myopathy (NM), a rare congenital myopathy linked to defects in proteins associated with thin filament structure and regulation. cDNA studies confirmed pathogenic consequences of the splice variant, eliciting exon-skipping and intron retention events leading to a frameshift. Western blot showed deficiency of troponin-Tfast protein with secondary loss of troponin-Ifast . We establish a homozygous splice variant in TNNT3 as the likely cause of severe congenital NM with distal arthrogryposis, characterized by specific involvement of Type-2 fibers and deficiency of troponin-Tfast .
Keywords: TNNT3; genetics; nemaline myopathy; neuromuscular disease; troponin T-fast.
© 2017 Wiley Periodicals, Inc.
Figures

References
-
- Ju Y, Li J, Xie C, Ritchlin CT, Xing L, Hilton MJ, Schwarz EM. Troponin T3 expression in skeletal and smooth muscle is required for growth and postnatal survival: characterization of Tnnt3(tm2a(KOMP)Wtsi) mice. Genesis (New York, NY: 2000) 2013;51(9):667–675. doi: 10.1002/dvg.22407. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

