Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues
- PMID: 29267188
- PMCID: PMC5943969
- DOI: 10.3390/molecules23010009
Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues
Abstract
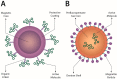
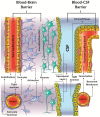
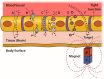
One of the most challenging goals in pharmacological research is overcoming the Blood Brain Barrier (BBB) to deliver drugs to the Central Nervous System (CNS). The use of physical means, such as steady and alternating magnetic fields to drive nanocarriers with proper magnetic characteristics may prove to be a useful strategy. The present review aims at providing an up-to-date picture of the applications of magnetic-driven nanotheranostics agents to the CNS. Although well consolidated on physical ground, some of the techniques described herein are still under investigation on in vitro or in silico models, while others have already entered in-or are close to-clinical validation. The review provides a concise overview of the physical principles underlying the behavior of magnetic nanoparticles (MNPs) interacting with an external magnetic field. Thereafter we describe the physiological pathways by which a substance can reach the brain from the bloodstream and then we focus on those MNP applications that aim at a nondestructive crossing of the BBB such as static magnetic fields to facilitate the passage of drugs and alternating magnetic fields to increment BBB permeability by magnetic heating. In conclusion, we briefly cite the most notable biomedical applications of MNPs and some relevant remarks about their safety and potential toxicity.
Keywords: blood brain barrier; central nervous system; delivery; magnetic nanoparticles; targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Movement of magnetic nanoparticles in brain tissue: mechanisms and impact on normal neuronal function.Nanomedicine. 2015 Oct;11(7):1821-9. doi: 10.1016/j.nano.2015.06.003. Epub 2015 Jun 24. Nanomedicine. 2015. PMID: 26115639 Free PMC article.
-
Simulation of magnetic nanoparticles crossing through a simplified blood-brain barrier model for Glioblastoma multiforme treatment.Comput Methods Programs Biomed. 2021 Nov;212:106477. doi: 10.1016/j.cmpb.2021.106477. Epub 2021 Oct 19. Comput Methods Programs Biomed. 2021. PMID: 34736172
-
Transmigration of magnetite nanoparticles across the blood-brain barrier in a rodent model: influence of external and alternating magnetic fields.Nanoscale. 2022 Dec 8;14(47):17589-17606. doi: 10.1039/d2nr02210a. Nanoscale. 2022. PMID: 36409463
-
Pharmaceutical Targeting of the Brain.Curr Pharm Des. 2016;22(35):5442-5462. doi: 10.2174/1381612822666160726144203. Curr Pharm Des. 2016. PMID: 27464716 Review.
-
Strategies for transporting nanoparticles across the blood-brain barrier.Biomater Sci. 2016 Feb;4(2):219-29. doi: 10.1039/c5bm00383k. Biomater Sci. 2016. PMID: 26646694 Review.
Cited by
-
Nanomedicine in Neuroprotection, Neuroregeneration, and Blood-Brain Barrier Modulation: A Narrative Review.Medicina (Kaunas). 2024 Aug 24;60(9):1384. doi: 10.3390/medicina60091384. Medicina (Kaunas). 2024. PMID: 39336425 Free PMC article. Review.
-
A Historical Review of Brain Drug Delivery.Pharmaceutics. 2022 Jun 16;14(6):1283. doi: 10.3390/pharmaceutics14061283. Pharmaceutics. 2022. PMID: 35745855 Free PMC article. Review.
-
Beyond Oncological Hyperthermia: Physically Drivable Magnetic Nanobubbles as Novel Multipurpose Theranostic Carriers in the Central Nervous System.Molecules. 2020 Apr 30;25(9):2104. doi: 10.3390/molecules25092104. Molecules. 2020. PMID: 32365941 Free PMC article.
-
A Review of the Neuroprotective Properties of Exosomes Derived from Stem Cells and Exosome-Coated Nanoparticles for Treating Neurodegenerative Diseases and Stroke.Int J Mol Sci. 2025 Apr 21;26(8):3915. doi: 10.3390/ijms26083915. Int J Mol Sci. 2025. PMID: 40332773 Free PMC article. Review.
-
Magneto-Mechanical Actuation Induces Endothelial Permeability.ACS Biomater Sci Eng. 2023 Dec 11;9(12):6902-6914. doi: 10.1021/acsbiomaterials.3c01571. Epub 2023 Nov 28. ACS Biomater Sci Eng. 2023. PMID: 38014849 Free PMC article.
References
-
- World Health Organization (WHO) Neurological Disorders: Public Health Challenges. WHO Press; Geneva, Switzerland: 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

