Impact of diabetes on gingival wound healing via oxidative stress
- PMID: 29267310
- PMCID: PMC5739411
- DOI: 10.1371/journal.pone.0189601
Impact of diabetes on gingival wound healing via oxidative stress
Abstract
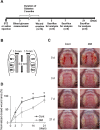
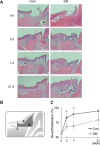
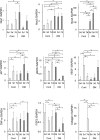
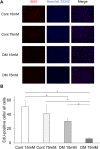
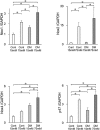
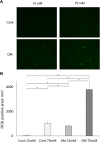
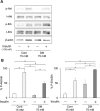
The aim of this study is to investigate the mechanisms linking high glucose to gingival wound healing. Bilateral wounds were created in the palatal gingiva adjacent to maxillary molars of control rats and rats with streptozotocin-induced diabetes. After evaluating postsurgical wound closure by digital imaging, the maxillae including wounds were resected for histological examinations. mRNA expressions of angiogenesis, inflammation, and oxidative stress markers in the surgical sites were quantified by real-time polymerase chain reaction. Primary fibroblast culture from the gingiva of both rats was performed in high glucose and normal medium. In vitro wound healing and cell proliferation assays were performed. Oxidative stress marker mRNA expressions and reactive oxygen species production were measured. Insulin resistance was evaluated via PI3K/Akt and MAPK/Erk signaling following insulin stimulation using Western blotting. To clarify oxidative stress involvement in high glucose culture and cells of diabetic rats, cells underwent N-acetyl-L-cysteine treatment; subsequent Akt activity was measured. Wound healing in diabetic rats was significantly delayed compared with that in control rats. Nox1, Nox2, Nox4, p-47, and tumor necrosis factor-α mRNA levels were significantly higher at baseline in diabetic rats than in control rats. In vitro study showed that cell proliferation and migration significantly decreased in diabetic and high glucose culture groups compared with control groups. Nox1, Nox2, Nox4, and p47 expressions and reactive oxygen species production were significantly higher in diabetic and high glucose culture groups than in control groups. Akt phosphorylation decreased in the high glucose groups compared with the control groups. Erk1/2 phosphorylation increased in the high glucose groups, with or without insulin treatment, compared with the control groups. Impaired Akt phosphorylation partially normalized after antioxidant N-acetyl-L-cysteine treatment. Thus, delayed gingival wound healing in diabetic rats occurred because of impaired fibroblast proliferation and migration. Fibroblast dysfunction may occur owing to high glucose-induced insulin resistance via oxidative stress.
Conflict of interest statement
Figures




Similar articles
-
High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts.PLoS One. 2018 Aug 9;13(8):e0201855. doi: 10.1371/journal.pone.0201855. eCollection 2018. PLoS One. 2018. PMID: 30092096 Free PMC article.
-
In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and cytotoxicity/genotoxicity profile.Food Chem Toxicol. 2015 Jul;81:54-61. doi: 10.1016/j.fct.2015.04.001. Epub 2015 Apr 3. Food Chem Toxicol. 2015. PMID: 25846499
-
Metformin accelerates wound healing by Akt phosphorylation of gingival fibroblasts in insulin-resistant prediabetes mice.J Periodontol. 2022 Feb;93(2):256-268. doi: 10.1002/JPER.21-0362. Epub 2021 Sep 23. J Periodontol. 2022. PMID: 34427916
-
Nrf2 Activation in Keratinocytes: A Central Role in Diabetes-Associated Wound Healing.Exp Dermatol. 2024 Oct;33(10):e15189. doi: 10.1111/exd.15189. Exp Dermatol. 2024. PMID: 39373525 Review.
-
The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing.Oxid Med Cell Longev. 2021 Feb 4;2021:8852759. doi: 10.1155/2021/8852759. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33628388 Free PMC article. Review.
Cited by
-
Ganoderma Microsporum Immunomodulatory Protein Alleviates Inflammaging and Oxidative Stress in Diabetes-Associated Periodontitis via Nrf2 Signaling Activation: An In Vitro Study.Antioxidants (Basel). 2024 Jul 8;13(7):817. doi: 10.3390/antiox13070817. Antioxidants (Basel). 2024. PMID: 39061886 Free PMC article.
-
Recent advances in responsive hydrogels for diabetic wound healing.Mater Today Bio. 2022 Dec 1;18:100508. doi: 10.1016/j.mtbio.2022.100508. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36504542 Free PMC article. Review.
-
Aster koraiensis extract improves impaired skin wound healing during hyperglycemia.Integr Med Res. 2018 Dec;7(4):351-357. doi: 10.1016/j.imr.2018.09.001. Epub 2018 Sep 22. Integr Med Res. 2018. PMID: 30591889 Free PMC article.
-
Human Gingival Fibroblasts Exposed to Extremely Low-Frequency Electromagnetic Fields: In Vitro Model of Wound-Healing Improvement.Int J Mol Sci. 2019 Apr 29;20(9):2108. doi: 10.3390/ijms20092108. Int J Mol Sci. 2019. PMID: 31035654 Free PMC article.
-
Anti-inflammaging effects of vitamin D in human gingival fibroblasts with advanced glycation end product stimulation.J Dent Sci. 2023 Apr;18(2):666-673. doi: 10.1016/j.jds.2022.10.003. Epub 2022 Oct 26. J Dent Sci. 2023. PMID: 37021258 Free PMC article.
References
-
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20. doi: 10.1038/414813a - DOI - PubMed
-
- King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79(8 Suppl):1527–34. doi: 10.1902/jop.2008.080246 - DOI - PubMed
-
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7(12):738–48. doi: 10.1038/nrendo.2011.106 - DOI - PubMed
-
- Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol. 2013;84(4 Suppl):S135–52. doi: 10.1902/jop.2013.1340013 - DOI - PubMed
-
- Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015(11):CD004714 doi: 10.1002/14651858.CD004714.pub3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

