Refined sgRNA efficacy prediction improves large- and small-scale CRISPR-Cas9 applications
- PMID: 29267886
- PMCID: PMC5814880
- DOI: 10.1093/nar/gkx1268
Refined sgRNA efficacy prediction improves large- and small-scale CRISPR-Cas9 applications
Abstract
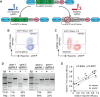
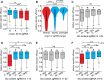
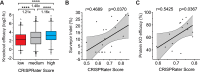
Genome editing with the CRISPR-Cas9 system has enabled unprecedented efficacy for reverse genetics and gene correction approaches. While off-target effects have been successfully tackled, the effort to eliminate variability in sgRNA efficacies-which affect experimental sensitivity-is in its infancy. To address this issue, studies have analyzed the molecular features of highly active sgRNAs, but independent cross-validation is lacking. Utilizing fluorescent reporter knock-out assays with verification at selected endogenous loci, we experimentally quantified the target efficacies of 430 sgRNAs. Based on this dataset we tested the predictive value of five recently-established prediction algorithms. Our analysis revealed a moderate correlation (r = 0.04 to r = 0.20) between the predicted and measured activity of the sgRNAs, and modest concordance between the different algorithms. We uncovered a strong PAM-distal GC-content-dependent activity, which enabled the exclusion of inactive sgRNAs. By deriving nine additional predictive features we generated a linear model-based discrete system for the efficient selection (r = 0.4) of effective sgRNAs (CRISPRater). We proved our algorithms' efficacy on small and large external datasets, and provide a versatile combined on- and off-target sgRNA scanning platform. Altogether, our study highlights current issues and efforts in sgRNA efficacy prediction, and provides an easily-applicable discrete system for selecting efficient sgRNAs.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

