Drp1 Mitochondrial Fission in D1 Neurons Mediates Behavioral and Cellular Plasticity during Early Cocaine Abstinence
- PMID: 29268097
- PMCID: PMC5747376
- DOI: 10.1016/j.neuron.2017.11.037
Drp1 Mitochondrial Fission in D1 Neurons Mediates Behavioral and Cellular Plasticity during Early Cocaine Abstinence
Abstract
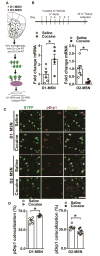
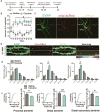
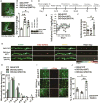
Altered brain energy homeostasis is a key adaptation occurring in the cocaine-addicted brain, but the effect of cocaine on the fundamental source of energy, mitochondria, is unknown. We demonstrate an increase of dynamin-related protein-1 (Drp1), the mitochondrial fission mediator, in nucleus accumbens (NAc) after repeated cocaine exposure and in cocaine-dependent individuals. Mdivi-1, a demonstrated fission inhibitor, blunts cocaine seeking and locomotor sensitization, while blocking c-Fos induction and excitatory input onto dopamine receptor-1 (D1) containing NAc medium spiny neurons (MSNs). Drp1 and fission promoting Drp1 are increased in D1-MSNs, consistent with increased smaller mitochondria in D1-MSN dendrites after repeated cocaine. Knockdown of Drp1 in D1-MSNs blocks drug seeking after cocaine self-administration, while enhancing the fission promoting Drp1 enhances seeking after long-term abstinence from cocaine. We demonstrate a role for altered mitochondrial fission in the NAc, during early cocaine abstinence, suggesting potential therapeutic treatment of disrupting mitochondrial fission in cocaine addiction.
Keywords: Drp1; cocaine; medium spiny neurons; mitochondria; nucleus accumbens; self-administration.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors report no biomedical financial interests or potential conflicts of interest.
Figures





References
-
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical. Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization. Dev Cell. 2008;14:193–204. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

