Insulin Concentration in Vials Randomly Purchased in Pharmacies in the United States: Considerable Loss in the Cold Supply Chain
- PMID: 29268624
- PMCID: PMC6134310
- DOI: 10.1177/1932296817747292
Insulin Concentration in Vials Randomly Purchased in Pharmacies in the United States: Considerable Loss in the Cold Supply Chain
Abstract
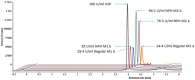
The insulin content in vials/cartridges should be 95 U/ml upon release. No independent confirmation of insulin concentration is available when purchased in the pharmacy (end of the cold supply chain). We quantitatively measured intact insulin in randomly acquired multidose human insulin vials by standard analytical methods. Eighteen 10 ml vials from two manufacturers (M1 and M2) were randomly acquired. The intact insulin concentration ranged from 13.9 to 94.2 U/ml, mean 40.2 U/ml. No vial met the minimum standard of 95 U/ml. These results imply the cold supply chain impacts insulin concentrations to a larger extent than anticipated. Patients are paying high prices for insulin and should expect to receive insulin vials with adequate insulin content in return.
Keywords: analysis; insulin; insulin content; quality assurance; vials.
Conflict of interest statement
Figures

Comment in
-
Lilly Calls into Question the Validity of Published Insulin Concentration Results.J Diabetes Sci Technol. 2018 Jul;12(4):892-893. doi: 10.1177/1932296818762502. Epub 2018 Mar 7. J Diabetes Sci Technol. 2018. PMID: 29514507 Free PMC article. No abstract available.
-
In Response to Carter and Heinemann: Insulin Concentration in Vials Randomly Purchased in Pharmacies in the United States: Considerable Loss in the Cold Supply Chain.J Diabetes Sci Technol. 2018 Jul;12(4):890-891. doi: 10.1177/1932296818761972. Epub 2018 Mar 16. J Diabetes Sci Technol. 2018. PMID: 29546770 Free PMC article. No abstract available.
References
-
- Chambers EE, Legido-Quigley C, Smith N, Fountain KJ. Development of a fast method for direct analysis of intact synthetic insulins in human plasma: the large peptide challenge. Bioanalysis. 2013;5(1):65-81. - PubMed
-
- Congress.gov. H.R.3204—Drug Quality and Security Act. Available at: https://www.congress.gov/bill/113th-congress/house-bill/3204. Accessed September 18, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

