Phase I clinical trial of a novel autologous modified-DC vaccine in patients with resected NSCLC
- PMID: 29268708
- PMCID: PMC5740508
- DOI: 10.1186/s12885-017-3859-3
Phase I clinical trial of a novel autologous modified-DC vaccine in patients with resected NSCLC
Abstract
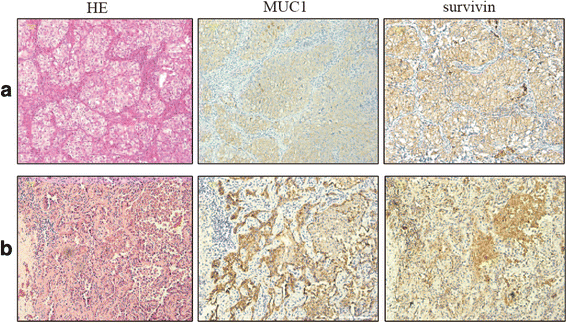
Background: The primary aim of this study was to evaluate the safety of a novel dendritic cell (DC) vaccine pulsed with survivin and MUC1, silenced with suppressor of cytokine signaling 1 (SOCS1), and immune stimulated with flagellin for patients with stage I to IIIA non-small cell lung cancer (NSCLC) in a phase I open-label, uncontrolled, and dose-escalation trial. Moreover, we evaluate the potential efficacy of this modified DC vaccine as secondary aim.
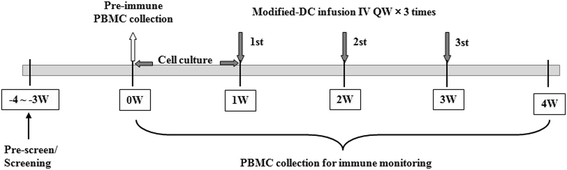
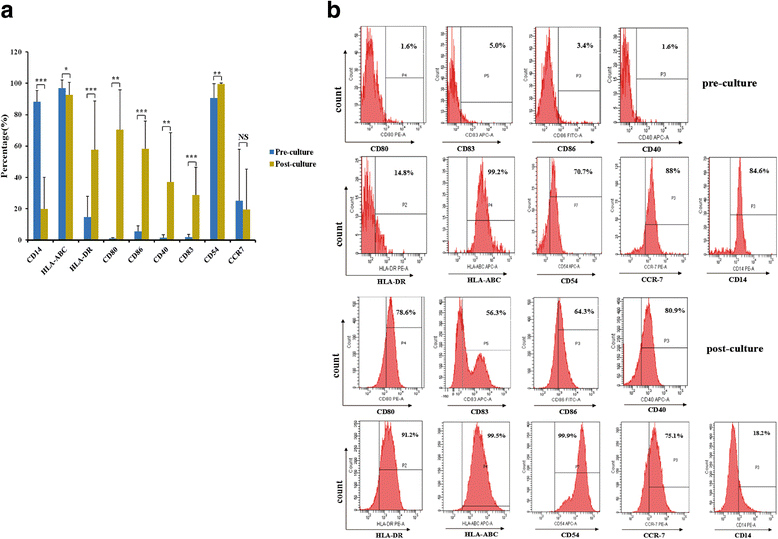
Methods: The patients were treated with the vaccine at 1 × 106, 1 × 107and the maximum dose 8 × 107 at day 7, 14, and 21 after characterization of the vaccine phenotype by flow cytometry. The safety of the vaccine was assessed by adverse events, and the efficacy by the levels of several specific tumor markers and the patient quality of life.
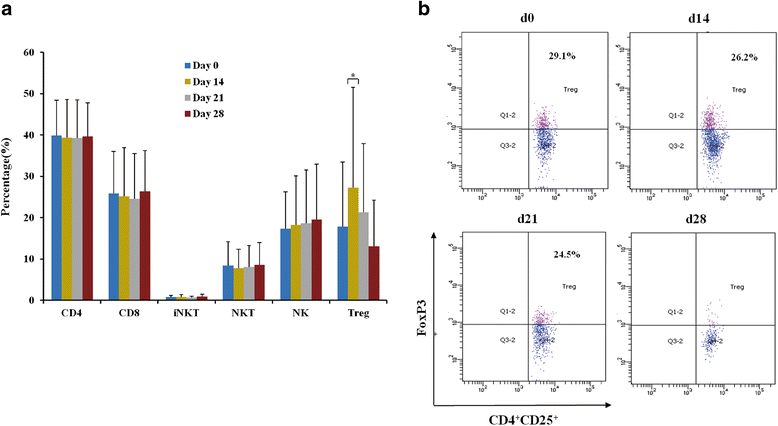
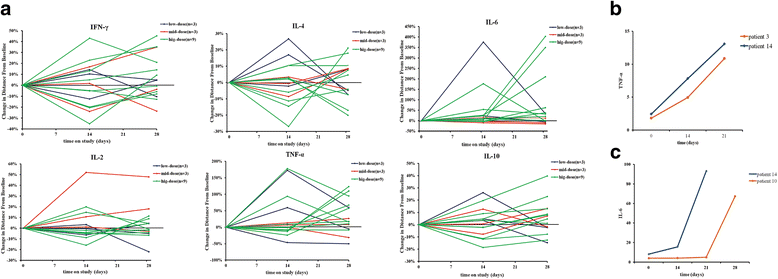
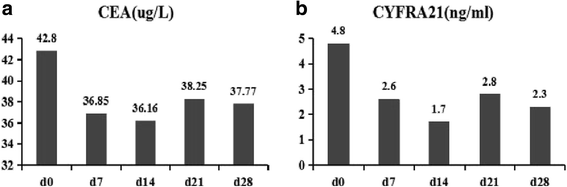
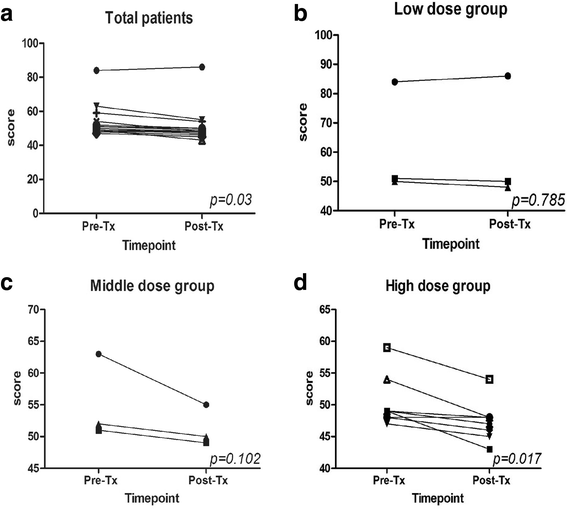
Results: The vaccine was well tolerated without dose-limiting toxicity even at higher doses. The most common adverse event reported was just grade 1 flu-like symptoms without unanticipated or serious adverse event. A significant decrease in CD3 + CD4 + CD25 + Foxp3+ T regulatory (Treg) cell number and increase in TNF-α and IL-6 were observed in two patients. Two patients showed 15% and 64% decrease in carcino-embryonic antigen and CYFRA21, respectively. The vaccination with the maximum dose significantly improved the patients'quality of life when administered at the highest dose. More importantly, in the long-term follow-up until February 17, 2017, 1 patient had no recurrence, 1 patients had a progressive disease (PD), and 1 patient was died in the low dose group. In the middle dose group, all 3 patients had no recurrence. In the high dose group, 1 patient was died, 1 patient had a PD, and the other 7 patients had no recurrence.
Conclusions: We provide preliminary data on the safety and efficacy profile of a novel vaccine against non-small cell lung cancer, which was reasonably well tolerated, induced modest antitumor activity without dose-limiting toxicity, and improved patients' quality of life. Further more, the vaccine maybe a very efficacious treatment for patients with resected NSCLC to prevent recurrence. Our findings on the safety and efficacy of the vaccine in this phase I trial warrant future phase II/III clinical trial.
Keywords: Modified-DCvaccine; Non-small cell lung cancer (NSCLC); Phase I clinical trial.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the ethics committee of the Third Affiliated Hospital of Kunming Medical University and was carried out in accordance with criteria of Good Clinical Practice.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Phase II Study of Immunotherapy With Tecemotide and Bevacizumab After Chemoradiation in Patients With Unresectable Stage III Non-Squamous Non-Small-Cell Lung Cancer (NS-NSCLC): A Trial of the ECOG-ACRIN Cancer Research Group (E6508).Clin Lung Cancer. 2020 Nov;21(6):520-526. doi: 10.1016/j.cllc.2020.06.007. Epub 2020 Jun 12. Clin Lung Cancer. 2020. PMID: 32807654 Free PMC article. Clinical Trial.
-
Assessing PDL-1 and PD-1 in Non-Small Cell Lung Cancer: A Novel Immunoscore Approach.Clin Lung Cancer. 2017 Mar;18(2):220-233.e8. doi: 10.1016/j.cllc.2016.09.009. Epub 2016 Oct 5. Clin Lung Cancer. 2017. PMID: 27816392
-
Impact of dendritic cell vaccines pulsed with Wilms' tumour-1 peptide antigen on the survival of patients with advanced non-small cell lung cancers.Eur J Cancer. 2013 Mar;49(4):852-9. doi: 10.1016/j.ejca.2012.11.005. Epub 2012 Dec 11. Eur J Cancer. 2013. PMID: 23245331
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
-
INGN-225: a dendritic cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: observed association between immune response and enhanced chemotherapy effect.Expert Opin Biol Ther. 2010 Jun;10(6):983-91. doi: 10.1517/14712598.2010.484801. Expert Opin Biol Ther. 2010. PMID: 20420527 Free PMC article. Review.
Cited by
-
Clinical relevance and therapeutic aspects of professional antigen-presenting cells in lung cancer.Med Oncol. 2022 Sep 29;39(12):237. doi: 10.1007/s12032-022-01841-6. Med Oncol. 2022. PMID: 36175603 Review.
-
Dendritic Cell-Based Immunotherapy in Lung Cancer.Front Immunol. 2021 Feb 12;11:620374. doi: 10.3389/fimmu.2020.620374. eCollection 2020. Front Immunol. 2021. PMID: 33679709 Free PMC article. Review.
-
Characteristics of tumor microenvironment and novel immunotherapeutic strategies for non-small cell lung cancer.J Natl Cancer Cent. 2022 Oct 20;2(4):243-262. doi: 10.1016/j.jncc.2022.10.002. eCollection 2022 Dec. J Natl Cancer Cent. 2022. PMID: 39036549 Free PMC article. Review.
-
Progress in the development of cancer vaccines for lung cancer utilizing dendritic cells (Review).Oncol Lett. 2025 Apr 14;29(6):298. doi: 10.3892/ol.2025.15044. eCollection 2025 Jun. Oncol Lett. 2025. PMID: 40276084 Free PMC article. Review.
-
Mucin1 as a potential molecule for cancer immunotherapy and targeted therapy.J Cancer. 2024 Jan 1;15(1):54-67. doi: 10.7150/jca.88261. eCollection 2024. J Cancer. 2024. PMID: 38164273 Free PMC article. Review.
References
-
- Garcia M, Jemal A, Ward E, Center M, Hao Y, Siegel R, Thun M. Global Cancer Facts & Figures 2007. Atlanta. 2007;Т2007(1):52.
-
- Rami-Porta R, Crowley JJ, Goldstraw P. Review the revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:5. - PubMed
-
- Scagliotti GV, Parikh P, Von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Phase III. Study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non–small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

