Sub-chronic inhalation of lead oxide nanoparticles revealed their broad distribution and tissue-specific subcellular localization in target organs
- PMID: 29268755
- PMCID: PMC5740755
- DOI: 10.1186/s12989-017-0236-y
Sub-chronic inhalation of lead oxide nanoparticles revealed their broad distribution and tissue-specific subcellular localization in target organs
Abstract
Background: Lead is well known environmental pollutant, which can cause toxic effects in multiple organ systems. However, the influence of lead oxide nanoparticles, frequently emitted to the environment by high temperature technological processes, is still concealed. Therefore, we investigate lead oxide nanoparticle distribution through the body upon their entry into lungs and determine the microscopic and ultramicroscopic changes caused by the nanoparticles in primary and secondary target organs.
Methods: Adult female mice (ICR strain) were continuously exposed to lead oxide nanoparticles (PbO-NPs) with an average concentration approximately 106 particles/cm3 for 6 weeks (24 h/day, 7 days/week). At the end of the exposure period, lung, brain, liver, kidney, spleen, and blood were collected for chemical, histological, immunohistochemical and electron microscopic analyses.
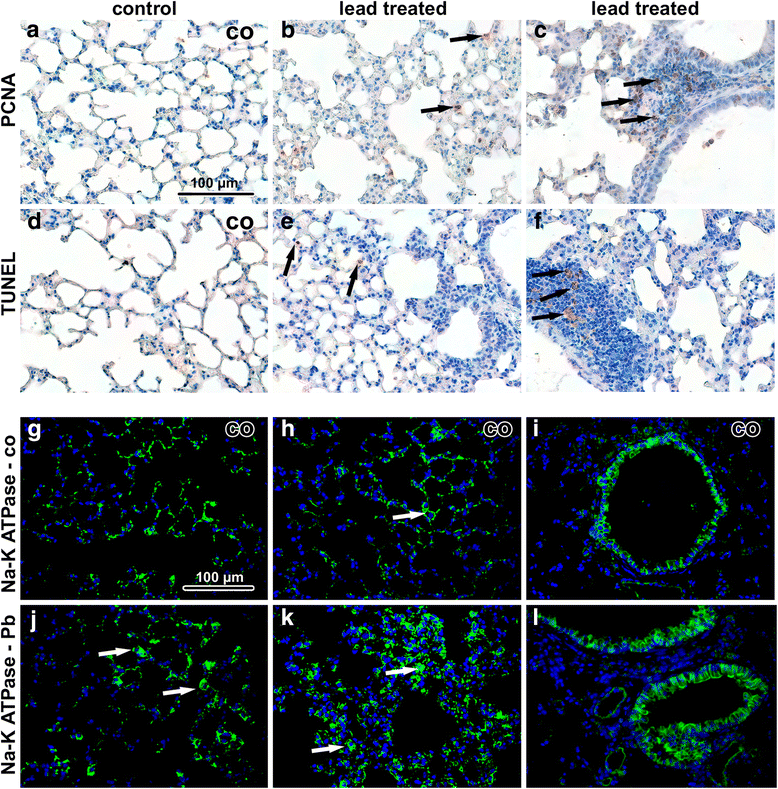
Results: Lead content was found to be the highest in the kidney and lungs, followed by the liver and spleen; the smallest content of lead was found in brain. Nanoparticles were located in all analysed tissues and their highest number was found in the lung and liver. Kidney, spleen and brain contained lower number of nanoparticles, being about the same in all three organs. Lungs of animals exposed to lead oxide nanoparticles exhibited hyperaemia, small areas of atelectasis, alveolar emphysema, focal acute catarrhal bronchiolitis and also haemostasis with presence of siderophages in some animals. Nanoparticles were located in phagosomes or formed clusters within cytoplasmic vesicles. In the liver, lead oxide nanoparticle exposure caused hepatic remodeling with enlargement and hydropic degeneration of hepatocytes, centrilobular hypertrophy of hepatocytes with karyomegaly, areas of hepatic necrosis, occasional periportal inflammation, and extensive accumulation of lipid droplets. Nanoparticles were accumulated within mitochondria and peroxisomes forming aggregates enveloped by an electron-dense mitochondrial matrix. Only in some kidney samples, we observed areas of inflammatory infiltrates around renal corpuscles, tubules or vessels in the cortex. Lead oxide nanoparticles were dispersed in the cytoplasm, but not within cell organelles. There were no significant morphological changes in the spleen as a secondary target organ. Thus, pathological changes correlated with the amount of nanoparticles found in cells rather than with the concentration of lead in a given organ.
Conclusions: Sub-chronic exposure to lead oxide nanoparticles has profound negative effects at both cellular and tissue levels. Notably, the fate and arrangement of lead oxide nanoparticles were dependent on the type of organs.
Keywords: Brain; Electron microscopy; Inhalation; Kidney; Lead oxide; Liver; Lung; Nanoparticles; Spleen; Toxicity.
Conflict of interest statement
Ethics approval
The experiments were performed in accordance with the ethics policy of the Institute of Animal Physiology and Genetics (no. 081/2010).
Consent for publication
The manuscript as submitted has been read and approved by all authors.
Competing interests
The authors declared that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures









References
-
- Sobanska S, Ricq N, Laboudigue A, Guillermo R, Bremard C, Laureyns J, Merlin J, Wignacourt J. Microchemical investigations of dust emitted by a lead smelter. Environmental Science & Technology. 1999;33:1334–1339. doi: 10.1021/es9805270. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

