Comparison of the efficacy of three topical antiseptic solutions for the prevention of catheter colonization: a multicenter randomized controlled study
- PMID: 29268759
- PMCID: PMC5740719
- DOI: 10.1186/s13054-017-1890-z
Comparison of the efficacy of three topical antiseptic solutions for the prevention of catheter colonization: a multicenter randomized controlled study
Abstract
Background: To compare the efficacy of three antiseptic solutions [0.5%, and 1.0% alcohol/chlorhexidine gluconate (CHG), and 10% aqueous povidone-iodine (PVI)] for the prevention of intravascular catheter colonization, we conducted a randomized controlled trial in patients from 16 intensive care units in Japan.
Methods: Adult patients undergoing central venous or arterial catheter insertions were randomized to have one of three antiseptic solutions applied during catheter insertion and dressing changes. The primary endpoint was the incidence of catheter colonization, and the secondary endpoint was the incidence of catheter-related bloodstream infections (CRBSI).
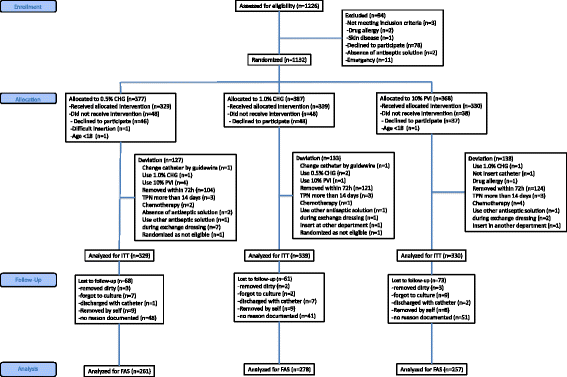
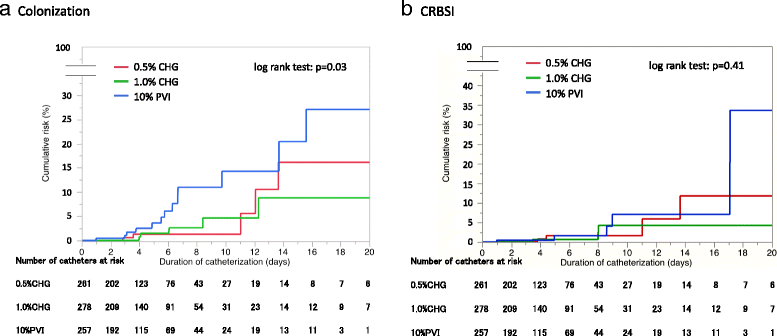
Results: Of 1132 catheters randomized, 796 (70%) were included in the full analysis set. Catheter-tip colonization incidence was 3.7, 3.9, and 10.5 events per 1000 catheter-days in 0.5% CHG, 1% CHG, and PVI groups, respectively (p = 0.03). Pairwise comparisons of catheter colonization between groups showed a significantly higher catheter colonization risk in the PVI group (0.5% CHG vs. PVI: hazard ratio, HR 0.33 [95% confidence interval, CI 0.12-0.95], p = 0.04; 1.0% CHG vs. PVI: HR 0.35 [95% CI 0.13-0.93], p = 0.04). Sensitivity analyses including all patients by multiple imputations showed consistent quantitative conclusions (0.5% CHG vs. PVI: HR 0.34, p = 0.03; 1.0% CHG vs. PVI: HR 0.35, p = 0.04). No significant differences were observed in the incidence of CRBSI between groups.
Conclusions: Both 0.5% and 1.0% alcohol CHG are superior to 10% aqueous PVI for the prevention of intravascular catheter colonization.
Trial registration: Japanese Primary Registries Network; No.: UMIN000008725 Registered on 1 September 2012.
Keywords: Anti-bacterial agents; Anti-infective agents; Catheter-related infections; Catheters; Chlorhexidine; Local; Povidone-iodine.
Conflict of interest statement
Ethics approval and consent to participate
The review boards of all participating institutions approved the study protocol, and patients/close relatives provided written informed consent. The specific names of all ethical bodies that approved this study in various participating sites involved in Additional file 1: Table S15.
Consent for publication
Not applicable
Competing interests
HY received lecture fees from Maruishi Pharmaceutical Company. MS received lecture fees from Maruishi Pharmaceutical Company. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- JANIS surveillance data of the incidence of CRBSI from January 2015 to June 2015. JANIS website. http://www.nih-janis.jp/report/open_report/2015/2/3/ICU_Open_Report_2015.... Accessed 20 Nov 2016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

