HtrA1 Mediated Intracellular Effects on Tubulin Using a Polarized RPE Disease Model
- PMID: 29269042
- PMCID: PMC5828370
- DOI: 10.1016/j.ebiom.2017.12.011
HtrA1 Mediated Intracellular Effects on Tubulin Using a Polarized RPE Disease Model
Abstract
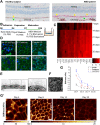
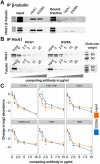
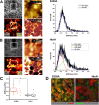
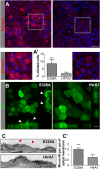
Age-related macular degeneration (AMD) is the leading cause of irreversible vision loss. The protein HtrA1 is enriched in retinal pigment epithelial (RPE) cells isolated from AMD patients and in drusen deposits. However, it is poorly understood how increased levels of HtrA1 affect the physiological function of the RPE at the intracellular level. Here, we developed hfRPE (human fetal retinal pigment epithelial) cell culture model where cells fully differentiated into a polarized functional monolayer. In this model, we fine-tuned the cellular levels of HtrA1 by targeted overexpression. Our data show that HtrA1 enzymatic activity leads to intracellular degradation of tubulin with a corresponding reduction in the number of microtubules, and consequently to an altered mechanical cell phenotype. HtrA1 overexpression further leads to impaired apical processes and decreased phagocytosis, an essential function for photoreceptor survival. These cellular alterations correlate with the AMD phenotype and thus highlight HtrA1 as an intracellular target for therapeutic interventions towards AMD treatment.
Keywords: Age-related macular degeneration; Cell stiffness; Disease modelling; HtrA serine peptidase 1; Mechanical properties; Phagocytic activity; Polarized human retinal, pigmented epithelium.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Augustin A., Lamerz J., Meistermann H., Golling S., Scheiblich S., Hermann J.C., Duchateau-Nguyen G., Tzouros M., Avila D.W., Langen H. Quantitative chemical proteomics profiling differentiates erlotinib from gefitinib in EGFR wild-type non-small cell lung carcinoma cell lines. Mol. Cancer Ther. 2013;12:520–529. - PubMed
-
- Barturen G., Rueda A., Hamberg M., Alganza A., Lebron R., Kotsyfakis M., Bu-Jun S., Kopper-Lalic D., Hackenberg M. sRNAbench: profiling of small RNAs and its sequence variants in single or multi-species high-throughput experiments. De Gruyter Open. 2014;1:21–31.
-
- Booij J.C., Baas D.C., Beisekeeva J., Gorgels T.G., Bergen A.A. The dynamic nature of Bruch's membrane. Prog. Retin. Eye Res. 2010;29:1–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

