A Numb-Mdm2 fuzzy complex reveals an isoform-specific involvement of Numb in breast cancer
- PMID: 29269425
- PMCID: PMC5800818
- DOI: 10.1083/jcb.201709092
A Numb-Mdm2 fuzzy complex reveals an isoform-specific involvement of Numb in breast cancer
Abstract
Numb functions as an oncosuppressor by inhibiting Notch signaling and stabilizing p53. This latter effect depends on the interaction of Numb with Mdm2, the E3 ligase that ubiquitinates p53 and commits it to degradation. In breast cancer (BC), loss of Numb results in a reduction of p53-mediated responses including sensitivity to genotoxic drugs and maintenance of homeostasis in the stem cell compartment. In this study, we show that the Numb-Mdm2 interaction represents a fuzzy complex mediated by a short Numb sequence encompassing its alternatively spliced exon 3 (Ex3), which is necessary and sufficient to inhibit Mdm2 and prevent p53 degradation. Alterations in the Numb splicing pattern are critical in BC as shown by increased chemoresistance of tumors displaying reduced levels of Ex3-containing isoforms, an effect that could be mechanistically linked to diminished p53 levels. A reduced level of Ex3-less Numb isoforms independently predicts poor outcome in BCs harboring wild-type p53. Thus, we have uncovered an important mechanism of chemoresistance and progression in p53-competent BCs.
© 2018 Colaluca et al.
Figures

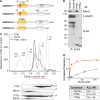
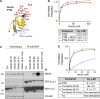


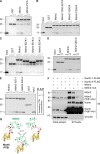




Comment in
-
Rewired Notch/p53 by Numb'ing Mdm2.J Cell Biol. 2018 Feb 5;217(2):445-446. doi: 10.1083/jcb.201712007. Epub 2018 Jan 16. J Cell Biol. 2018. PMID: 29339436 Free PMC article.
References
-
- Adams, P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.-W., Kapral G.J., Grosse-Kunstleve R.W., et al. . 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

