Recurrent infection progressively disables host protection against intestinal inflammation
- PMID: 29269445
- PMCID: PMC5824721
- DOI: 10.1126/science.aao5610
Recurrent infection progressively disables host protection against intestinal inflammation
Abstract
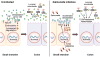
Intestinal inflammation is the central pathological feature of colitis and the inflammatory bowel diseases. These syndromes arise from unidentified environmental factors. We found that recurrent nonlethal gastric infections of Gram-negative Salmonella enterica Typhimurium (ST), a major source of human food poisoning, caused inflammation of murine intestinal tissue, predominantly the colon, which persisted after pathogen clearance and irreversibly escalated in severity with repeated infections. ST progressively disabled a host mechanism of protection by inducing endogenous neuraminidase activity, which accelerated the molecular aging and clearance of intestinal alkaline phosphatase (IAP). Disease was linked to a Toll-like receptor 4 (TLR4)-dependent mechanism of IAP desialylation with accumulation of the IAP substrate and TLR4 ligand, lipopolysaccharide-phosphate. The administration of IAP or the antiviral neuraminidase inhibitor zanamivir was therapeutic by maintaining IAP abundance and function.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures







Comment in
-
Infectious Scarring: Setting the Trigger for Intestinal Inflammation.Cell Host Microbe. 2018 Feb 14;23(2):154-156. doi: 10.1016/j.chom.2018.01.017. Cell Host Microbe. 2018. PMID: 29447693
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

