Group VII Ethylene Response Factors in Arabidopsis: Regulation and Physiological Roles
- PMID: 29269576
- PMCID: PMC5813551
- DOI: 10.1104/pp.17.01225
Group VII Ethylene Response Factors in Arabidopsis: Regulation and Physiological Roles
Abstract
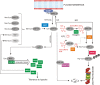
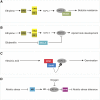
The role of ERF-VII TFs in higher plants is to coordinate their signature response to oxygen deficiency, but additional layers of modulation of ERF-VII activity enrich their regulatory range.
Figures



References
-
- Aicart-Ramos C, Valero RA, Rodriguez-Crespo I (2011) Protein palmitoylation and subcellular trafficking. Biochim Biophys Acta 1808: 2981–2994 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

