A molecular basis for water motion detection by the mechanosensory lateral line of zebrafish
- PMID: 29269857
- PMCID: PMC5740173
- DOI: 10.1038/s41467-017-01604-2
A molecular basis for water motion detection by the mechanosensory lateral line of zebrafish
Abstract
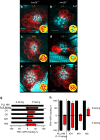
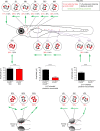
Detection of water motion by the lateral line relies on mechanotransduction complexes at stereocilia tips. This sensory system is comprised of neuromasts, patches of hair cells with stereociliary bundles arranged with morphological mirror symmetry that are mechanically responsive to two opposing directions. Here, we find that transmembrane channel-like 2b (Tmc2b) is differentially required for mechanotransduction in the zebrafish lateral line. Despite similarities in neuromast hair cell morphology, three classes of these cells can be distinguished by their Tmc2b reliance. We map mechanosensitivity along the lateral line using imaging and electrophysiology to determine that a hair cell's Tmc2b dependence is governed by neuromast topological position and hair bundle orientation. Overall, water flow is detected by molecular machinery that can vary between hair cells of different neuromasts. Moreover, hair cells within the same neuromast can break morphologic symmetry of the sensory organ at the stereocilia tips.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







References
-
- Montgomery J, Baker C, Carton A. The lateral line can mediate rheotaxis in fish. Nature. 1997;389:960–963. doi: 10.1038/40135. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

