Risk factors for the discontinuation of roflumilast in patients with chronic obstructive pulmonary disease
- PMID: 29270006
- PMCID: PMC5720347
- DOI: 10.2147/COPD.S143967
Risk factors for the discontinuation of roflumilast in patients with chronic obstructive pulmonary disease
Abstract
Introduction: Roflumilast is a phosphodiesterase-4 inhibitor, which can decrease exacerbation in patients with chronic obstructive pulmonary disease (COPD). However, adverse effects are a major barrier to medication use, and little is known regarding the risk factors for discontinuation of roflumilast in COPD patients.
Method: A search of the clinical databases identified all patients who were prescribed roflumilast between December 2012 and April 2015 in the four hospitals of The Catholic University of Korea, Korea. The study subjects were limited to patients who had taken 500 μg of roflumilast. We studied the factors associated with drug discontinuation and drug adverse events by univariate and multivariate analyses.
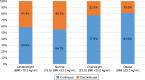
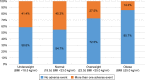
Results: Among 154 eligible patients, 54 (35.1%) discontinued their roflumilast prescription. Most patients were elderly, male, current or former smokers, and had moderate-to-severe airflow limitation. Low-body mass index (BMI) patients were more likely to undergo drug discontinuation (1-unit decrease in BMI: odds ratio [OR] =1.165, p=0.006; BMI <23 kg/m2: OR =2.960, p=0.004). Fifty-five patients (35.7%) had adverse events. Loss of appetite, diarrhea, nausea, headache, and weight loss were the most frequent adverse events. Low-BMI patients were more likely to experience adverse events (1-unit decrease in BMI: OR =1.151, p=0.010; BMI <23 kg/m2: OR =2.644, p=0.009).
Conclusions: The patient discontinuation and adverse events rates in this study were higher than in previous randomized controlled studies. Discontinuation of roflumilast in ethnic Koreans is more likely to occur in low-BMI patients. In a clinical setting, low-BMI patients can more easily discontinue roflumilast; clinicians should, therefore, provide greater care for these patients.
Keywords: adverse event; body mass index; chronic obstructive pulmonary disease; phosphodiesterase-4 inhibitor.
Conflict of interest statement
Disclosure CKR received consulting and/or lecture fees from MSD, AstraZeneca, Novartis, GSK, Takeda, Mundipharma, Sandoz, Boehringer-Ingelheim, and Teva-Handok. The authors report no other conflicts of interest in this work.
Figures

References
-
- Tashkin DP. Roflumilast: the new orally active, selective phophodiesterase-4 inhibitor, for the treatment of COPD. Expert Opin Pharmacother. 2014;15:85–96. - PubMed
-
- Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast–an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–571. - PubMed
-
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

