Common founder effects of hereditary hemochromatosis, Wilson´s disease, the long QT syndrome and autosomal recessive deafness caused by two novel mutations in the WHRN and TMC1 genes
- PMID: 29270100
- PMCID: PMC5735936
- DOI: 10.1186/s41065-017-0052-2
Common founder effects of hereditary hemochromatosis, Wilson´s disease, the long QT syndrome and autosomal recessive deafness caused by two novel mutations in the WHRN and TMC1 genes
Abstract
Background: Genealogy and molecular genetic studies of a Swedish river valley population resulted in a large pedigree, showing that the hereditary hemochromatosis (HH) HFE/p.C282Y mutation is inherited with other recessive disorders such as Wilson´s disease (WND), a rare recessive disorder of copper overload. The population also contain individuals with the Swedish long QT syndrome (LQTS1) founder mutation (KCNQ1/p.Y111C) which in homozygotes causes the Jervell & Lange Nielsen syndrome (JLNS) and hearing loss (HL).Aims of the study were to test whether the Swedish long QT founder mutation originated in an ancestral HFE family and if carriers had an increased risk for hemochromatosis (HH), a treatable disorder. We also aimed to identify the pathogenic mutation causing the hearing loss disorder segregating in the pedigree.
Methods: LQTS patients were asked about their ancestry and possible origin in a HH family. They were also offered a predictive testing for the HFE genotype. Church books were screened for families with hearing loss. One HH family had two members with hearing loss, who underwent molecular genetic analysis of the LQTS founder mutation, connexin 26 and thereafter exome sequencing. Another family with hearing loss in repeat generations was also analyzed for connexin 26 and underwent exome sequencing.
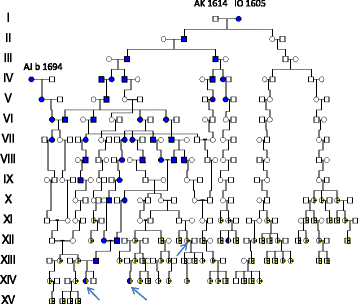
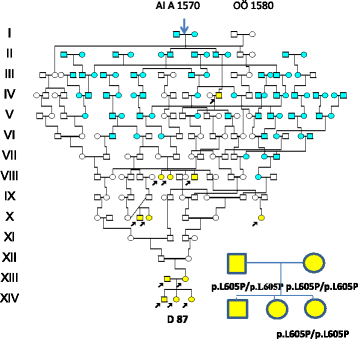
Results: Of nine LQTS patients studied, four carried a HFE mutation (two p.C282Y, two p.H63D), none was homozygous. Three LQTS patients confirmed origin in a female founder ( b 1694, identical to AJ b 1694, a HFE pedigree member from the Fax river. Her descent of 44 HH families, included also 29 families with hearing loss (HL) suggesting JLNS. Eleven LQTS probands confirmed origin in a second founder couple (b 1614/1605) in which the woman b 1605 was identical to a HFE pedigree member from the Fjällsjö river. In her descent there were not only 64 HH, six WND families, one JLNS, but also 48 hearing loss families. Most hearing loss was non syndromic and caused by founder effects of the late 16th century. One was of Swedish origin carrying the WHRN, c.1977delC, (p.S660Afs*30) mutation, the other was a TMC1(NM_138691),c.1814T>C,(p.L605P) mutation, possibly of Finnish origin.
Conclusions: Deep human HFE genealogies show HFE to be associated with other genetic disorders like Wilson´s disease, LQTS, JLNS, and autosomal recessive hearing loss. Two new homozygous HL mutations in WHRN/p.S660Afs*30 and TMC1/p.L605P were identified,none of them previously reported from Scandinavia. The rarity of JLNS was possibly caused by miscarriage or intrauterine death. Most hearing loss (81.7%) was seen after 1844 when first cousin marriages were permitted. However, only 10 (10.3%) came from 1st cousin unions and only 2 (2.0 %) was born out of wedlock.
Keywords: DFNB31; Hereditary hemochromatosis; Jervell and Lange- Nielsen´s syndrome; Long QT syndrome; Non syndromic hearing loss; TMC1; WHRN; Wilson´s disease.
Conflict of interest statement
The study was approved by the Regional Ethical Review Board at the University of Göteborg, (Dnr 834/14) ,T 214/16 and T1076-16). Consent for publication of hearing loss and LQTS was obtained.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Olsson KS, Ritter B, Raha-Chowdhury R. HLA-A3-B14 and the origin of the haemochromatosis C282Y mutation: founder effects and recombination events during 12 generations in a Scandinavian family with major iron overload. Eur J Haematol. 2010;84(2):145–53.3. doi: 10.1111/j.1600-0609.2009.01376.x. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

