Adenosine A2A Receptor Deletion Blocks the Beneficial Effects of Lactobacillus reuteri in Regulatory T-Deficient Scurfy Mice
- PMID: 29270168
- PMCID: PMC5723640
- DOI: 10.3389/fimmu.2017.01680
Adenosine A2A Receptor Deletion Blocks the Beneficial Effects of Lactobacillus reuteri in Regulatory T-Deficient Scurfy Mice
Abstract
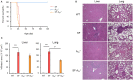
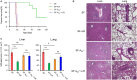
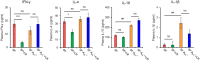
The lack of a functional Foxp3 transcription factor and regulatory T (Treg) cells causes lethal, CD4+ T cell-driven autoimmune diseases in scurfy (SF) mice and humans. Recent studies have shown that adenosine A2A receptor activation limits inflammation and tissue damage, thereby playing an anti-inflammatory role. However, the role of the adenosine A2A receptor in the development of disease in SF mice remains unclear. Using a genetic approach, we found that adenosine A2A receptor deletion in SF mice (SF[Formula: see text]) does not affect early life events, the development of a lymphoproliferative disorder, or hyper-production of pro-inflammatory cytokines seen in the Treg-deficiency state. As shown previously, Lactobacillus reuteri DSM 17938 treatment prolonged survival and reduced multiorgan inflammation in SF mice. In marked contrast, A2A receptor deletion completely blocked these beneficial effects of L. reuteri in SF mice. Altogether, these results suggest that although absence of the adenosine A2A receptor does not affect the development of disease in SF mice, it plays a critical role in the immunomodulation by L. reuteri in Treg-deficiency disease. The adenosine A2A receptor and its activation may have a role in treating other Treg dysfunction-mediated autoimmune diseases.
Keywords: IPEX; Lactobacillus reuteri; adenosine A2A receptor; autoimmunity; cytokines; probiotic; regulatory T deficiency; scurfy.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

