Placental Histopathology and Clinical Presentation of Severe Congenital Zika Syndrome in a Human Immunodeficiency Virus-Exposed Uninfected Infant
- PMID: 29270171
- PMCID: PMC5725436
- DOI: 10.3389/fimmu.2017.01704
Placental Histopathology and Clinical Presentation of Severe Congenital Zika Syndrome in a Human Immunodeficiency Virus-Exposed Uninfected Infant
Abstract
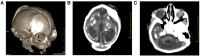
In the large Zika virus (ZIKV) epidemic that occurred in Brazil in 2015, the intrauterine fetal exposure to ZIKV was associated with a significant risk of developing microcephaly and neurological disorders in the infected infants. ZIKV-associated disease has since been reported in 24 countries in the Americas. At present, definitive evidence is lacking regarding the intrauterine co-exposure to ZIKV and other viral infections and whether the coinfection impacts the risk of acquiring either infection or disease severity. Here, we provide evidence of intrauterine exposure to both ZIKV and human immunodeficiency virus (HIV) infections, causing congenital Zika syndrome in an HIV-exposed uninfected infant. Clinical, imaging and laboratory examinations of the pregnant woman and the newborn were performed. Histopathology, ZIKV/HIV-specific immunoassays, and ultrastructural evaluation of the placenta were performed. The Zika-asymptomatic, HIV-positive pregnant woman underwent ultrasounds revealing fetal cerebral ventriculomegaly, microcephaly, and brain atrophy. Her baby girl was born small for gestational age and with the neurological sequelae of congenital Zika syndrome. The evaluation of the abnormally large term placenta revealed severe damage to the maternal decidua and chorionic villi, cells positive for ZIKV-specific antigens but not for HIV antigens, and intracellular membranous clusters of virus-like particles approximately 25 nm in diameter. The rapid progression and severity of the congenital Zika syndrome may be related to the uncontrolled HIV disease in the mother. The poor inflammatory response observed in the placenta may have reduced the inherent risk of mother-to-child transmission of HIV.
Keywords: Zika virus; congenital Zika syndrome; histopathology; human immunodeficiency virus; microcephaly; placenta.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

