Protein Translocation into the Intermembrane Space and Matrix of Mitochondria: Mechanisms and Driving Forces
- PMID: 29270408
- PMCID: PMC5725982
- DOI: 10.3389/fmolb.2017.00083
Protein Translocation into the Intermembrane Space and Matrix of Mitochondria: Mechanisms and Driving Forces
Abstract
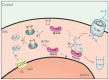
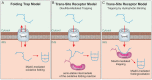
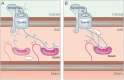
Mitochondria contain two aqueous subcompartments, the matrix and the intermembrane space (IMS). The matrix is enclosed by both the inner and outer mitochondrial membranes, whilst the IMS is sandwiched between the two. Proteins of the matrix are synthesized in the cytosol as preproteins, which contain amino-terminal matrix targeting sequences that mediate their translocation through translocases embedded in the outer and inner membrane. For these proteins, the translocation reaction is driven by the import motor which is part of the inner membrane translocase. The import motor employs matrix Hsp70 molecules and ATP hydrolysis to ratchet proteins into the mitochondrial matrix. Most IMS proteins lack presequences and instead utilize the IMS receptor Mia40, which facilitates their translocation across the outer membrane in a reaction that is coupled to the formation of disulfide bonds within the protein. This process requires neither ATP nor the mitochondrial membrane potential. Mia40 fulfills two roles: First, it acts as a holdase, which is crucial in the import of IMS proteins and second, it functions as a foldase, introducing disulfide bonds into newly imported proteins, which induces and stabilizes their natively folded state. For several Mia40 substrates, oxidative folding is an essential prerequisite for their assembly into oligomeric complexes. Interestingly, recent studies have shown that the two functions of Mia40 can be experimentally separated from each other by the use of specific mutants, hence providing a powerful new way to dissect the different physiological roles of Mia40. In this review we summarize the current knowledge relating to the mitochondrial matrix-targeting and the IMS-targeting/Mia40 pathway. Moreover, we discuss the mechanistic properties by which the mitochondrial import motor on the one hand and Mia40 on the other, drive the translocation of their substrates into the organelle. We propose that the lateral diffusion of Mia40 in the inner membrane and the oxidation-mediated folding of incoming polypeptides supports IMS import.
Keywords: Mia40; brownian ratchet; disulfide bond; foldase; holdase; mitochondria; protein import.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

