Safety and Efficacy of Combination ACTHar Gel and Tacrolimus in Treatment-Resistant Focal Segmental Glomerulosclerosis and Membranous Glomerulopathy
- PMID: 29270498
- PMCID: PMC5733765
- DOI: 10.1016/j.ekir.2017.05.015
Safety and Efficacy of Combination ACTHar Gel and Tacrolimus in Treatment-Resistant Focal Segmental Glomerulosclerosis and Membranous Glomerulopathy
Abstract
Introduction: H.P. ACTHar gel is a preparation of melanocortin peptides that has been used to treat resistant forms of nephrotic syndrome. To determine whether combination therapy with ACTHar gel and tacrolimus reduces proteinuria and stabilizes renal function, we conducted a prospective, open-label trial in patients with treatment-resistant membranous glomerulopathy (MGN) and focal segmental glomerulosclerosis (FSGS).
Methods: Nine patients with treatment-resistant MGN and 13 with treatment-resistant FSGS received subcutaneous ACTHar gel for 6 months. Patients with no response or a partial response to ACTHar gel alone received an additional 6 months of therapy with combination ACTHar gel and oral tacrolimus. The study endpoint was the percentage of patients achieving a complete or partial remission after 6 months of combination therapy.
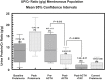
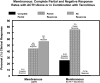
Results: Among patients with MGN, treatment with ACTHar gel alone achieved a partial remission in 44% and no response in 56% of patients. No patient achieved a complete response with ACTHar gel therapy alone. An additional 6 months of combination therapy with ACTHar gel and tacrolimus resulted in partial and complete response rates of 25% and 75%, respectively. Among patients with FSGS, ACTHar gel therapy alone resulted in complete and partial response rate of 7.7% and 62.0%. Combination therapy increased complete response rates to 17% and partial responses to 66%. Proteinuria (urinary protein-to-creatinine ratio) was significantly reduced in both patients with MGN and those with FSGS after 6 months of ACTHar gel alone and was further reduced among the patients with MGN with the addition of tacrolimus. There were no significant changes in estimated glomerular filtration rate during the treatment phase or long-term follow-up.
Discussion: Combination therapy with ACTHar gel and tacrolimus was well tolerated by patients with treatment-resistant MGN and FSGS and significantly reduced proteinuria and improved clinical response rates compared with ACTHar gel alone.
Keywords: ACTHar gel; focal segmental glomerulosclerosis; membranous glomerulopathy; proteinuria.
Figures





References
-
- Korbet S.M. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23:1769–1776. - PubMed
-
- Ponticelli C., Rizzoni, Edefonti A. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int. 1993;43:1377–1384. - PubMed
-
- Cattran D.C., Appel G.B., Hebert L.A. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int. 1999;56:2220–2226. - PubMed
-
- Choudhry S., Bagga A., Hari P. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: a randomized controlled trial. Am J Kidney Dis. 2009;53:760–769. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

