Tolerability of Aquaretic-Related Symptoms Following Tolvaptan for Autosomal Dominant Polycystic Kidney Disease: Results From TEMPO 3:4
- PMID: 29270521
- PMCID: PMC5733681
- DOI: 10.1016/j.ekir.2017.07.004
Tolerability of Aquaretic-Related Symptoms Following Tolvaptan for Autosomal Dominant Polycystic Kidney Disease: Results From TEMPO 3:4
Abstract
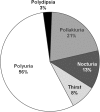
Introduction: In the randomized placebo-controlled Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) 3:4 trial, tolvaptan slowed kidney growth and renal function decline in subjects with autosomal dominant polycystic kidney disease (ADPKD). Consistent with its primary pharmacologic activity, tolvaptan use was commonly associated with aquaretic adverse events (AAEs) attributable to excess free water clearance.
Methods: A post hoc analysis of tolvaptan-related discontinuations from the pivotal randomized controlled trial TEMPO 3:4 and its open-label extension TEMPO 4:4.
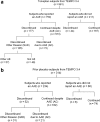
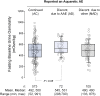
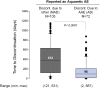
Results: In total, 750 of 961 tolvaptan-treated subjects (78%) in TEMPO 3:4 reported at least one AAE. Of these 750 subjects, 72 (10%) discontinued because of an AAE (aquaretic-discontinued group) and 573 (76%) continued (aquaretic-continued group). The aquaretic-discontinued subjects were younger, had better baseline renal function, and had higher fasting urine osmolality than aquaretic-continued subjects. Of the 750 subjects reporting an AAE, 105 (14%) discontinued for another reason (non-aquaretic-discontinued group). Compared to non-aquaretic-discontinued subjects, aquaretic-discontinued subjects were more commonly male, had better baseline renal function, and discontinued the study drug faster. After 3 years of therapy, 75% of tolvaptan subjects indicated that they could tolerate their current dose for the rest of their lives, compared to 85% of placebo subjects. These findings were corroborated by results in the open-label extension trial TEMPO 4:4.
Discussion: In this study, AAEs were common but well tolerated in ADPKD patients on tolvaptan. ADPKD patients in earlier stages of disease progression may be more sensitive to aquaretic symptoms, which may help in guiding tolvaptan dosing and titration decisions in the future.
Keywords: aquaretic adverse events; autosomal dominant polycystic kidney disease; discontinuation; drug safety; tolerability; tolvaptan.
Figures


References
-
- Grantham J.J. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–1485. - PubMed
-
- Torres V.E., Harris P.C., Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. - PubMed
-
- Grantham J.J., Mulamalla S., Swenson-Fields K.I. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7:556–566. - PubMed
-
- Ong A.C., Devuyst O., Knebelmann B. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385:1993–2002. - PubMed
-
- United States Renal Data System: 2014 annual data report: an overview of the epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014.
LinkOut - more resources
Full Text Sources
Other Literature Sources

