Assessing 'No Evidence of Disease Activity' Status in Patients with Relapsing-Remitting Multiple Sclerosis Receiving Fingolimod in Routine Clinical Practice: A Retrospective Analysis of the Multiple Sclerosis Clinical and Magnetic Resonance Imaging Outcomes in the USA (MS-MRIUS) Study
- PMID: 29270772
- PMCID: PMC5843701
- DOI: 10.1007/s40263-017-0482-4
Assessing 'No Evidence of Disease Activity' Status in Patients with Relapsing-Remitting Multiple Sclerosis Receiving Fingolimod in Routine Clinical Practice: A Retrospective Analysis of the Multiple Sclerosis Clinical and Magnetic Resonance Imaging Outcomes in the USA (MS-MRIUS) Study
Abstract
Background: 'No evidence of disease activity' (NEDA), a composite measure of clinical and magnetic resonance imaging outcomes, provides a comprehensive assessment of disease activity, but is not extensively reported in clinical practice. NEDA-3 is defined as patients with no new/enlarged T2 or gadolinium-enhancing lesions, no relapses, and no disability progression (according to Expanded Disability Status Scale scores). NEDA-4 comprises the components of NEDA-3 and a fourth criterion of ≤ 0.4% annualized brain volume loss.
Objective: The objective of this study was to assess NEDA status among patients with relapsing-remitting multiple sclerosis receiving fingolimod in clinical practice.
Methods: Clinical and magnetic resonance imaging data were retrospectively collected from 590 patients who initiated fingolimod at 33 multiple sclerosis centers in the USA. Patients were required to have a magnetic resonance imaging scan in the 6 months before or 1 month after fingolimod initiation (index period) and in the 9-24 months after fingolimod initiation (post-index period). Magnetic resonance imaging data were systematically quantified at a centralized reading facility. The proportions of patients with NEDA-3 or NEDA-4 status during fingolimod treatment were assessed.
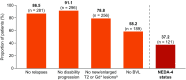
Results: During the follow-up period (median: 16 months), data to assess NEDA-3 and NEDA-4 were available for 586 and 325 patients, respectively. In the post-index period, 58.7% of patients achieved NEDA-3 status (no relapses, 85.2%; no new/enlarged T2/gadolinium-enhancing lesions, 76.3%; no disability progression, 87.9%) and 37.2% achieved NEDA-4 status (no relapses, 86.5%; no new/enlarged T2/gadolinium-enhancing lesions, 78.8%; no disability progression, 91.1%; brain volume loss ≤ 0.4, 58.2%).
Conclusion: Among patients receiving fingolimod, over half achieved NEDA-3 status and over one-third achieved NEDA-4 status.
Conflict of interest statement
Funding
This study was funded by Novartis Pharma AG. Oxford PharmaGenesis received funding from Novartis Pharma AG for medical writing support. IQVIA received funding from Novartis Pharma AG. The open access fee was covered by Novartis Pharma AG
Conflict of interest
Bianca Weinstock-Guttman has received honoraria as a speaker and consultant for Biogen, EMD Serono, Sanofi Genzyme, Novartis, Teva Pharmaceuticals, and Genentech. She has received research funds from Biogen, EMD Serono, Sanofi Genzyme, Novartis, and Teva Pharmaceuticals. Jennie Medin and Diego Silva are paid employees of Novartis Pharma AG, Basel, Switzerland. Nasreen Khan is a paid consultant for IQVIA, Basel, Switzerland. Jonathan R. Korn was a paid employee of IQVIA (previously QuintilesIMS), Burlington, MA, USA at the time of this study. Ellen Lathi has received honoraria as a speaker and consultant for Acorda, Biogen, Genzyme, Novartis, Teva Pharmaceuticals, and Genentech. Jason Silversteen has no conflicts of interest to report. Jonathan Calkwood has performed advisory, consultancy, and speaker activities for Acorda, Biogen, EMD Serono, Genzyme, Novartis, Roche, and Teva; and has received grant/research support from Biogen, Celgene, Genzyme, Novartis, and Roche. Robert Zivadinov has received personal compensation from EMD Serono, Celgene, Claret Medical, Novartis, and Sanofi Genzyme for speaking and consultancy. He has received financial support for research activities from Claret Medical, IMS Health, InteKrin-Coherus, Novartis, Sanofi Genzyme, and Teva Pharmaceuticals.
Informed consent
For this type of study, formal consent is not required.
Figures


References
-
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556. doi: 10.1016/S1474-4422(14)70049-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

