Surveillance of Antibiotic Use and Resistance in Intensive Care Units (SARI)
- PMID: 29271345
- PMCID: PMC5763000
- DOI: 10.3238/arztebl.2017.0858
Surveillance of Antibiotic Use and Resistance in Intensive Care Units (SARI)
Abstract
Background: The project entitled Surveillance of Antibiotic Use and Resistance in Intensive Care Units (SARI) was initiated in Germany in 2000. In this article, we describe developments in antibiotic use and resistance rates in the participating intensive care units over the years 2001-2015.
Methods: The intensive care units supplied monthly figures on patient days, antibiotic use (in defined daily doses, DDD), and resistance data for 13 pathogens. The density of antibiotic use per 1000 patient days was calculated on the basis of antibiotic use, DDD, and patient days, and the resistance density per 1000 patient days was calculated from the number of resistant pathogens.
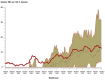
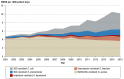
Results: In the years 2001-2015, data on 2 920 068 patient days were collected in 77 intensive care units. The average overall antibiotic use rose by 19% over this period, with a marked increase in the density of carbapenem use (from 76 to 250 DDD per 1000 patient days, +230%) and piperacillin-tazobactam use (from 42 to 146 DDD per 1000 patient days, +247%). The proportion of Escherichia coli and Klebsiella pneumoniae isolates that were resistant to third-generation cephalosporins increased markedly initially, then remained stable over the remainder of the observation period. The proportion of methicillin-resistant Staphylococcus aureus was stable over the entire period. The rates of vancomycin resistance among Enterococcus faecium isolates and imipenem resistance among gram-negative pathogens increased from 2.3% to 13.3% and from 0.1% to 0.3%, respectively.
Conclusion: The resistance density of gram-negative multiresistant pathogens in the participating intensive care units increased markedly. The rise in imipenem-resistant pathogens arouses particular concern. The increased use of broad-spectrum/reserve antibiotics may well have contributed to this development. Efforts to use antibiotics rationally, e.g., with the support of multidisciplinary "antibiotic stewardship" teams, are therefore vitally important. As participation in SARI is voluntary, these surveillance data cannot be considered representative of Germany as a whole.
Figures


Comment in
-
Changing Infection Patterns.Dtsch Arztebl Int. 2017 Dec 15;114(50):849-850. doi: 10.3238/arztebl.2017.0849. Dtsch Arztebl Int. 2017. PMID: 29271342 Free PMC article. No abstract available.
References
-
- Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14:742–750. - PubMed
-
- Holmes AH, Moore LS, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387:176–187. - PubMed
-
- Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–1098. - PubMed
-
- Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011) Diagn Microbiol Infect Dis. 2014;78:443–448. - PubMed
-
- Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

