Metal ion assisted interface re-engineering of a ferritin nanocage for enhanced biofunctions and cancer therapy
- PMID: 29271453
- PMCID: PMC5812362
- DOI: 10.1039/c7nr08188j
Metal ion assisted interface re-engineering of a ferritin nanocage for enhanced biofunctions and cancer therapy
Abstract
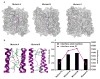
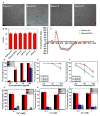
The bottom-up self-assembly of protein subunits into supramolecular nanoarchitectures is ubiquitously exploited to recapitulate and expand the features of natural proteins to advance nanoscience in medicine. Various chemical and biological re-engineering approaches are available to render diverse functions in the given proteins. They are, unfortunately, capable of compromising protein integrity and stability after extensive modifications. In this study, we introduce a new protein re-engineering method, metal ion assisted interface re-engineering (MAIR), to serve as a robust and universal strategy to extend the functions of self-assembly proteins by boosting structural features to advance their diverse biomedical applications. In particular, the MAIR strategy was applied to a widely used natural protein, ferritin, as a model protein to coordinate with copper ions in its mutagenic artificial metal binding domain. Structure directed rational protein mutagenesis was carried out at the C2 interface amino acid residues of the ferritin subunit for metal ion coordination site optimization. Copper binding at the artificial binding pocket was highly specific over the other divalent ions present in physiological fluids, and the structurally embedded copper ion in turn strengthened the overall protein integrity and stability. In the presence of isotopic copper-64, the interface re-engineered ferritin worked as a chelator-free molecular nanoprobe with an extraordinarily high specific activity to allow PET imaging of tumors in live animals. We also found that the re-engineered ferritin coordinating with copper ions demonstrates high drug loading capacity of a widely used anti-cancer agent, doxorubicin (DOX), to achieve significant drug retention at the tumor site and enhance tumor regression for improved anti-cancer effects. The MAIR approach, thus, exploited the copper ion to facilitate efficient one-step labeling of mutant ferritin derivatives for simultaneous molecular imaging and drug delivery. The reported interface re-engineering strategy provides an unparalleled opportunity to expand protein biofunctions to serve as a new theranostic agent in cancer research.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Re-engineering protein interfaces yields copper-inducible ferritin cage assembly.Nat Chem Biol. 2013 Mar;9(3):169-76. doi: 10.1038/nchembio.1163. Epub 2013 Jan 20. Nat Chem Biol. 2013. PMID: 23340339
-
Ferritin nanocages for early theranostics of tumors via inflammation-enhanced active targeting.Sci China Life Sci. 2022 Feb;65(2):328-340. doi: 10.1007/s11427-021-1976-0. Epub 2021 Aug 31. Sci China Life Sci. 2022. PMID: 34482518
-
Ferritin-based drug delivery systems: Hybrid nanocarriers for vascular immunotargeting.J Control Release. 2018 Jul 28;282:13-24. doi: 10.1016/j.jconrel.2018.02.042. Epub 2018 Mar 6. J Control Release. 2018. PMID: 29522833 Free PMC article. Review.
-
Re-engineering the inner surface of ferritin nanocage enables dual drug payloads for synergistic tumor therapy.Theranostics. 2022 Jan 24;12(4):1800-1815. doi: 10.7150/thno.68459. eCollection 2022. Theranostics. 2022. PMID: 35198074 Free PMC article.
-
Ferritin: A Multifunctional Nanoplatform for Biological Detection, Imaging Diagnosis, and Drug Delivery.Acc Chem Res. 2021 Sep 7;54(17):3313-3325. doi: 10.1021/acs.accounts.1c00267. Epub 2021 Aug 20. Acc Chem Res. 2021. PMID: 34415728 Review.
Cited by
-
Metal-Coordinated Supramolecular Self-Assemblies for Cancer Theranostics.Adv Sci (Weinh). 2021 Aug;8(16):e2101101. doi: 10.1002/advs.202101101. Epub 2021 Jun 18. Adv Sci (Weinh). 2021. PMID: 34145984 Free PMC article. Review.
-
Protein-Based Nanoparticles for the Imaging and Treatment of Solid Tumors: The Case of Ferritin Nanocages, a Narrative Review.Pharmaceutics. 2021 Nov 25;13(12):2000. doi: 10.3390/pharmaceutics13122000. Pharmaceutics. 2021. PMID: 34959283 Free PMC article. Review.
-
Polyphenol-based nanoplatform for MRI/PET dual-modality imaging guided effective combination chemotherapy.J Mater Chem B. 2019 Oct 7;7(37):5688-5694. doi: 10.1039/c9tb01597c. Epub 2019 Sep 2. J Mater Chem B. 2019. PMID: 31475276 Free PMC article.
-
Cargo loading within ferritin nanocages in preparation for tumor-targeted delivery.Nat Protoc. 2021 Oct;16(10):4878-4896. doi: 10.1038/s41596-021-00602-5. Epub 2021 Sep 8. Nat Protoc. 2021. PMID: 34497386 Review.
-
Identification of novel yolk ferritins unique to planarians: planarians supply aluminum rather than iron to vitellaria in egg capsules.Cell Tissue Res. 2021 Nov;386(2):391-413. doi: 10.1007/s00441-021-03506-8. Epub 2021 Jul 28. Cell Tissue Res. 2021. PMID: 34319433
References
-
- Fegan A, White B, Carlson JC, Wagner CR. Chem Rev. 2010;110:3315–3336. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

