Immunomodulatory activity of glycodelin: implications in allograft rejection
- PMID: 29271477
- PMCID: PMC5904719
- DOI: 10.1111/cei.13096
Immunomodulatory activity of glycodelin: implications in allograft rejection
Abstract
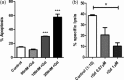
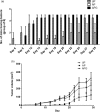
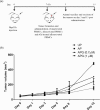
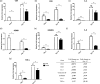
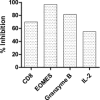
Glycodelin is an immunomodulator, indispensable for the maintenance of pregnancy in humans. The glycoprotein induces apoptosis in activated CD4+ T cells, monocytes and natural killer (NK) cells, and suppresses the activity of cytotoxic T cells, macrophages and dendritic cells. This study explores the immunosuppressive property of glycodelin for its possible use in preventing graft rejection. Because glycodelin is found only in certain primates, the hypothesis was investigated in an allograft nude mouse model. It is demonstrated that treatment of alloactivated mononuclear cells with glycodelin thwarts graft rejection. Glycodelin decreases the number of activated CD4+ and CD8+ cells and down-regulates the expression of key proteins known to be involved in graft demise such as granzyme-B, eomesodermin (EOMES), interleukin (IL)-2 and proinflammatory cytokines [tumour necrosis factor (TNF)-α and IL-6], resulting in a weakened cell-mediated immune response. Immunosuppressive drugs for treating allograft rejection are associated with severe side effects. Glycodelin, a natural immunomodulator in humans, would be an ideal alternative candidate.
Keywords: IL-2; T cells; granzyme-B; immunosuppression.
© 2017 British Society for Immunology.
Figures

Similar articles
-
Glycodelin-A interferes with IL-2/IL-2R signalling to induce cell growth arrest, loss of effector functions and apoptosis in T-lymphocytes.Hum Reprod. 2012 Apr;27(4):1005-15. doi: 10.1093/humrep/der477. Epub 2012 Feb 7. Hum Reprod. 2012. PMID: 22313865
-
Glycodelin regulates the numbers and function of peripheral natural killer cells.J Reprod Immunol. 2020 Feb;137:102625. doi: 10.1016/j.jri.2019.102625. Epub 2019 Oct 24. J Reprod Immunol. 2020. PMID: 31730930
-
Glycodelin A, an immunomodulatory protein in the endometrium, inhibits proliferation and induces apoptosis in monocytic cells.Int J Biochem Cell Biol. 2009 May;41(5):1138-47. doi: 10.1016/j.biocel.2008.10.009. Epub 2008 Oct 18. Int J Biochem Cell Biol. 2009. PMID: 18996219
-
Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade.Transplantation. 2017 Jan;101(1):32-44. doi: 10.1097/TP.0000000000001452. Transplantation. 2017. PMID: 27547870 Review.
-
[Immunopathology of cytomegalovirus pneumonia and allograft rejection in lung transplantation. Group of Pulmonary Transplantation of the University Paris-Sud].Rev Mal Respir. 1994;11(6):559-64. Rev Mal Respir. 1994. PMID: 7831505 Review. French.
Cited by
-
Polarization disorder of decidual NK cells in unexplained recurrent spontaneous abortion revealed by single-cell transcriptome analysis.Reprod Biol Endocrinol. 2022 Jul 27;20(1):108. doi: 10.1186/s12958-022-00980-9. Reprod Biol Endocrinol. 2022. PMID: 35897028 Free PMC article.
-
Coactosin-Like Protein 1 (COTL1) Could Be an Immunological and Prognostic Biomarker: From Pan-Cancer Analysis to Low-Grade Glioma Validation.J Inflamm Res. 2024 Mar 19;17:1805-1820. doi: 10.2147/JIR.S453509. eCollection 2024. J Inflamm Res. 2024. PMID: 38523681 Free PMC article.
-
Effect of Glycodelin on the Cytokine Profile of Rats during Allogeneic Bone Marrow Cell Transplantation.Bull Exp Biol Med. 2022 Sep;173(5):636-640. doi: 10.1007/s10517-022-05603-2. Epub 2022 Oct 10. Bull Exp Biol Med. 2022. PMID: 36210410
References
-
- Arck PC, Hecher K. Fetomaternal immune cross‐talk and its consequences for maternal and offspring's health. Nat Med 2013; 19:548–56. - PubMed
-
- Julkunen M, Rutanen EM, Koskimies A, Ranta T, Bohn H, Seppälä M. Distribution of placental protein 14 in tissues and body fluids during pregnancy. Br J Obstet Gynaecol 1985; 92:1145–51. - PubMed
-
- Dalton CF, Laird SM, Estdale SE, Saravelos HG, Li TC. Endometrial protein PP14 and CA‐125 in recurrent miscarriage patients; correlation with pregnancy outcome. Hum Reprod 1998; 13:3197–202. - PubMed
-
- Mukhopadhyay D, Sundereshan S, Rao C, Karande AA. Placental protein 14 induces apoptosis in T cells but not in monocytes. J Biol Chem 2001; 276:28268–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

