Differential mechanisms of adenosine- and ATPγS-induced microvascular endothelial barrier strengthening
- PMID: 29271489
- PMCID: PMC7273968
- DOI: 10.1002/jcp.26419
Differential mechanisms of adenosine- and ATPγS-induced microvascular endothelial barrier strengthening
Abstract
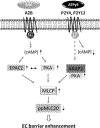
Maintenance of the endothelial cell (EC) barrier is critical to vascular homeostasis and a loss of barrier integrity results in increased vascular permeability. While the mechanisms that govern increased EC permeability have been under intense investigation over the past several decades, the processes regulating the preservation/restoration of the EC barrier remain poorly understood. Herein we show that the extracellular purines, adenosine (Ado) and adenosine 5'-[γ-thio]-triphosphate (ATPγS) can strengthen the barrier function of human lung microvascular EC (HLMVEC). This ability involves protein kinase A (PKA) activation and decreases in myosin light chain 20 (MLC20) phosphorylation secondary to the involvement of MLC phosphatase (MLCP). In contrast to Ado, ATPγS-induced PKA activation is accompanied by a modest, but significant decrease in cyclic adenosine monophosphate (cAMP) levels supporting the existence of an unconventional cAMP-independent pathway of PKA activation. Furthermore, ATPγS-induced EC barrier strengthening does not involve the Rap guanine nucleotide exchange factor 3 (EPAC1) which is directly activated by cAMP but is instead dependent upon PKA-anchor protein 2 (AKAP2) expression. We also found that AKAP2 can directly interact with the myosin phosphatase-targeting protein MYPT1 and that depletion of AKAP2 abolished ATPγS-induced increases in transendothelial electrical resistance. Ado-induced strengthening of the HLMVEC barrier required the coordinated activation of PKA and EPAC1 in a cAMP-dependent manner. In summary, ATPγS-induced enhancement of the EC barrier is EPAC1-independent and is instead mediated by activation of PKA which is then guided by AKAP2, in a cAMP-independent mechanism, to activate MLCP which dephosphorylates MLC20 resulting in reduced EC contraction and preservation.
Keywords: ATPγS; PKA; adenosine; endothelial barrier protection; myosin light chain.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
Figures








Similar articles
-
Extracellular beta-nicotinamide adenine dinucleotide (beta-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement.J Cell Physiol. 2010 Apr;223(1):215-23. doi: 10.1002/jcp.22029. J Cell Physiol. 2010. PMID: 20054824 Free PMC article.
-
cAMP/PKA antagonizes thrombin-induced inactivation of endothelial myosin light chain phosphatase: role of CPI-17.Cardiovasc Res. 2010 Jul 15;87(2):375-84. doi: 10.1093/cvr/cvq065. Epub 2010 Mar 3. Cardiovasc Res. 2010. PMID: 20202976
-
Rac GTPase is a hub for protein kinase A and Epac signaling in endothelial barrier protection by cAMP.Microvasc Res. 2010 Mar;79(2):128-38. doi: 10.1016/j.mvr.2009.11.007. Epub 2009 Dec 3. Microvasc Res. 2010. PMID: 19962392 Free PMC article.
-
Emerging themes of cAMP regulation of the pulmonary endothelial barrier.Am J Physiol Lung Cell Mol Physiol. 2011 May;300(5):L667-78. doi: 10.1152/ajplung.00433.2010. Epub 2011 Feb 18. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21335524 Free PMC article. Review.
-
Regulation of the inflammatory response of vascular endothelial cells by EPAC1.Br J Pharmacol. 2012 May;166(2):434-46. doi: 10.1111/j.1476-5381.2011.01808.x. Br J Pharmacol. 2012. PMID: 22145651 Free PMC article. Review.
Cited by
-
Adenosine and ATPγS protect against bacterial pneumonia-induced acute lung injury.Sci Rep. 2020 Oct 22;10(1):18078. doi: 10.1038/s41598-020-75224-0. Sci Rep. 2020. PMID: 33093565 Free PMC article.
-
Role of the Purinergic P2Y2 Receptor in Pulmonary Hypertension.Int J Environ Res Public Health. 2021 Oct 20;18(21):11009. doi: 10.3390/ijerph182111009. Int J Environ Res Public Health. 2021. PMID: 34769531 Free PMC article. Review.
-
Extracellular adenosine enhances pulmonary artery vasa vasorum endothelial cell barrier function via Gi/ELMO1/Rac1/PKA-dependent signaling mechanisms.Am J Physiol Cell Physiol. 2020 Jul 1;319(1):C183-C193. doi: 10.1152/ajpcell.00505.2019. Epub 2020 May 20. Am J Physiol Cell Physiol. 2020. PMID: 32432925 Free PMC article.
-
P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases.Int J Mol Sci. 2020 Sep 18;21(18):6855. doi: 10.3390/ijms21186855. Int J Mol Sci. 2020. PMID: 32962005 Free PMC article. Review.
-
Pannexin1: insight into inflammatory conditions and its potential involvement in multiple organ dysfunction syndrome.Front Immunol. 2023 Aug 30;14:1217366. doi: 10.3389/fimmu.2023.1217366. eCollection 2023. Front Immunol. 2023. PMID: 37711629 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

