A muscle-specific MuRF1-E2 network requires stabilization of MuRF1-E2 complexes by telethonin, a newly identified substrate
- PMID: 29271608
- PMCID: PMC5803617
- DOI: 10.1002/jcsm.12249
A muscle-specific MuRF1-E2 network requires stabilization of MuRF1-E2 complexes by telethonin, a newly identified substrate
Abstract
Background: Muscle wasting is observed in the course of many diseases and also during physiological conditions (disuse, ageing). Skeletal muscle mass is largely controlled by the ubiquitin-proteasome system and thus by the ubiquitinating enzymes (E2s and E3s) that target substrates for subsequent degradation. MuRF1 is the only E3 ubiquitin ligase known to target contractile proteins (α-actin, myosins) during catabolic situations. However, MuRF1 depends on E2 ubiquitin-conjugating enzymes for ubiquitin chain formation on the substrates. MuRF1-E2 couples are therefore putative targets for preventing muscle wasting.
Methods: We focused on 14 E2 enzymes that are either expressed in skeletal muscle or up-regulated during atrophying conditions. In this work, we demonstrated that only highly sensitive and complementary interactomic approaches (surface plasmon resonance, yeast three-hybrid, and split green fluorescent protein) allowed the identification of MuRF1 E2 partners.
Results: Five E2 enzymes physically interacted with MuRF1, namely, E2E1, E2G1, E2J1, E2J2, and E2L3. Moreover, we demonstrated that MuRF1-E2E1 and MuRF1-E2J1 interactions are facilitated by telethonin, a newly identified MuRF1 substrate. We next showed that the five identified E2s functionally interacted with MuRF1 since, in contrast to the non-interacting E2D2, their co-expression in HEK293T cells with MuRF1 led to increased telethonin degradation. Finally, we showed that telethonin governed the affinity between MuRF1 and E2E1 or E2J1.
Conclusions: We report here the first MuRF1-E2s network, which may prove valuable for deciphering the precise mechanisms involved in the atrophying muscle programme and for proposing new therapeutical approaches.
Keywords: E3 ubiquitin ligase; Tcap; UBE2; muscle wasting; split-GFP; ubiquitin-conjugating enzyme.
© 2017 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Figures
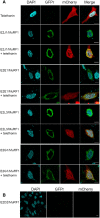
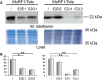
Similar articles
-
UBE2E1 Is Preferentially Expressed in the Cytoplasm of Slow-Twitch Fibers and Protects Skeletal Muscles from Exacerbated Atrophy upon Dexamethasone Treatment.Cells. 2018 Nov 16;7(11):214. doi: 10.3390/cells7110214. Cells. 2018. PMID: 30453501 Free PMC article.
-
UBE2D2 is not involved in MuRF1-dependent muscle wasting during hindlimb suspension.Int J Biochem Cell Biol. 2016 Oct;79:488-493. doi: 10.1016/j.biocel.2016.06.019. Epub 2016 Jul 1. Int J Biochem Cell Biol. 2016. PMID: 27378730
-
Structure predictions of MuRF1-UBE2 complexes identify amino acid residues governing interaction selectivity for each MuRF1-E2 pair.FEBS J. 2025 May;292(10):2559-2577. doi: 10.1111/febs.70017. Epub 2025 Feb 10. FEBS J. 2025. PMID: 39930652 Free PMC article.
-
Ubiquitin-protein ligases in muscle wasting.Int J Biochem Cell Biol. 2005 Oct;37(10):2088-97. doi: 10.1016/j.biocel.2004.11.010. Epub 2004 Dec 14. Int J Biochem Cell Biol. 2005. PMID: 16125112 Review.
-
A critical discussion on the relationship between E3 ubiquitin ligases, protein degradation, and skeletal muscle wasting: it's not that simple.Am J Physiol Cell Physiol. 2023 Dec 1;325(6):C1567-C1582. doi: 10.1152/ajpcell.00457.2023. Epub 2023 Nov 13. Am J Physiol Cell Physiol. 2023. PMID: 37955121 Free PMC article. Review.
Cited by
-
Emerging Strategies Targeting Catabolic Muscle Stress Relief.Int J Mol Sci. 2020 Jun 30;21(13):4681. doi: 10.3390/ijms21134681. Int J Mol Sci. 2020. PMID: 32630118 Free PMC article. Review.
-
Case Report: Two New Cases of Autosomal-Recessive Hypertrophic Cardiomyopathy Associated With TRIM63-Compound Heterozygous Variant.Front Genet. 2022 Feb 22;13:743472. doi: 10.3389/fgene.2022.743472. eCollection 2022. Front Genet. 2022. PMID: 35273634 Free PMC article.
-
UBE2E1 Is Preferentially Expressed in the Cytoplasm of Slow-Twitch Fibers and Protects Skeletal Muscles from Exacerbated Atrophy upon Dexamethasone Treatment.Cells. 2018 Nov 16;7(11):214. doi: 10.3390/cells7110214. Cells. 2018. PMID: 30453501 Free PMC article.
-
Silver linings on the horizon: highlights from the 10th Cachexia Conference.J Cachexia Sarcopenia Muscle. 2018 Feb;9(1):176-182. doi: 10.1002/jcsm.12290. J Cachexia Sarcopenia Muscle. 2018. PMID: 29417752 Free PMC article.
-
The Ubiquitin Proteasome System in Neuromuscular Disorders: Moving Beyond Movement.Int J Mol Sci. 2020 Sep 3;21(17):6429. doi: 10.3390/ijms21176429. Int J Mol Sci. 2020. PMID: 32899400 Free PMC article. Review.
References
-
- Vainzof M, Moreira ES, Suzuki OT, Faulkner G, Valle G, Beggs AH, et al. Telethonin protein expression in neuromuscular disorders. Biochim Biophys Acta BBA ‐ Mol Basis Dis 2002. Oct 9;1588:33–40. - PubMed
-
- Knöll R, Hoshijima M, Hoffman HM, Person V, Lorenzen‐Schmidt I, Bang M‐L, et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 2002. Dec 27;111:943–955. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

