Establishing in vitro in vivo correlations to screen monoclonal antibodies for physicochemical properties related to favorable human pharmacokinetics
- PMID: 29271699
- PMCID: PMC5825195
- DOI: 10.1080/19420862.2017.1417718
Establishing in vitro in vivo correlations to screen monoclonal antibodies for physicochemical properties related to favorable human pharmacokinetics
Abstract
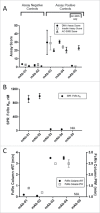
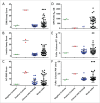
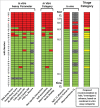
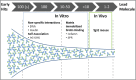
Implementation of in vitro assays that correlate with in vivo human pharmacokinetics (PK) would provide desirable preclinical tools for the early selection of therapeutic monoclonal antibody (mAb) candidates with minimal non-target-related PK risk. Use of these tools minimizes the likelihood that mAbs with unfavorable PK would be advanced into costly preclinical and clinical development. In total, 42 mAbs varying in isotype and soluble versus membrane targets were tested in in vitro and in vivo studies. MAb physicochemical properties were assessed by measuring non-specific interactions (DNA- and insulin-binding ELISA), self-association (affinity-capture self-interaction nanoparticle spectroscopy) and binding to matrix-immobilized human FcRn (surface plasmon resonance and column chromatography). The range of scores obtained from each in vitro assay trended well with in vivo clearance (CL) using both human FcRn transgenic (Tg32) mouse allometrically projected human CL and observed human CL, where mAbs with high in vitro scores resulted in rapid CL in vivo. Establishing a threshold value for mAb CL in human of 0.32 mL/hr/kg enabled refinement of thresholds for each in vitro assay parameter, and using a combinatorial triage approach enabled the successful differentiation of mAbs at high risk for rapid CL (unfavorable PK) from those with low risk (favorable PK), which allowed mAbs requiring further characterization to be identified. Correlating in vitro parameters with in vivo human CL resulted in a set of in vitro tools for use in early testing that would enable selection of mAbs with the greatest likelihood of success in the clinic, allowing costly late-stage failures related to an inadequate exposure profile, toxicity or lack of efficacy to be avoided.
Keywords: AC-SINS; FcRn binding; IgG; clearance, in vitro assays; mAb; monoclonal antibody, neonatal Fc receptor; pharmacokinetics; polyreactivity.
Figures


References
-
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, et al.. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol (Baltimore, Md. : 1950). 2003;170:3528–3533. doi:10.4049/jimmunol.170.7.3528. PMID:12646614. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
