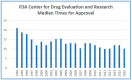
Facilitating a More Efficient Commercial Review Process for Pediatric Drugs and Biologics
- PMID: 29271878
- PMCID: PMC5871948
- DOI: 10.3390/diseases6010002
Facilitating a More Efficient Commercial Review Process for Pediatric Drugs and Biologics
Abstract
Over the past two decades, the biopharmaceutical industry has seen unprecedented expansion and innovation in concert with significant technological advancements. While the industry has experienced marked growth, the regulatory system in the United States still operates at a capacity much lower than the influx of new drug and biologic candidates. As a result, it has become standard for months or even years of waiting for commercial approval by the U.S. Food and Drug Administration. These regulatory delays have generated a system that stifles growth and innovation due to the exorbitant costs associated with awaiting approval from the nation's sole regulatory agency. The recent re-emergence of diseases that impact pediatric demographics represents one particularly acute reason for developing a regulatory system that facilitates a more efficient commercial review process. Herein, we present a range of initiatives that could represent early steps toward alleviating the delays in approving life-saving therapeutics.
Keywords: FDA; commercial drug approval; privatization; windfall tax.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Innovating by developing new uses of already-approved drugs: trends in the marketing approval of supplemental indications.Clin Ther. 2013 Jun;35(6):808-18. doi: 10.1016/j.clinthera.2013.04.004. Epub 2013 May 28. Clin Ther. 2013. PMID: 23726388
-
AMCP Partnership Forum: Enabling the Exchange of Clinical and Economic Information Pre-FDA Approval.J Manag Care Spec Pharm. 2017 Jan;23(1):105-112. doi: 10.18553/jmcp.2016.16366. Epub 2016 Dec 22. J Manag Care Spec Pharm. 2017. PMID: 28025919 Free PMC article.
-
Approval of Cancer Drugs With Uncertain Therapeutic Value: A Comparison of Regulatory Decisions in Europe and the United States.Milbank Q. 2020 Dec;98(4):1219-1256. doi: 10.1111/1468-0009.12476. Epub 2020 Oct 6. Milbank Q. 2020. PMID: 33021339 Free PMC article.
-
Biosimilars: impact of biologic product life cycle and European experience on the regulatory trajectory in the United States.Clin Ther. 2012 Feb;34(2):400-19. doi: 10.1016/j.clinthera.2011.12.005. Epub 2012 Jan 13. Clin Ther. 2012. PMID: 22244050 Review.
-
Follow-on biologics: competition in the biopharmaceutical marketplace.J Am Pharm Assoc (2003). 2006 Mar-Apr;46(2):193-201; quiz 202-4. doi: 10.1331/154434506776180595. J Am Pharm Assoc (2003). 2006. PMID: 16602229 Review.
References
-
- Kriz B., Fabianova K., Maixnerova M., Benes C., Maly M. Pertussis: A reemerging infection? Epidemiol. Mikrobiol. Imunol. Cas. Spol. Epidemiol. Mikrobiol. Ceske Lek. Spol. JE Purkyne. 2007;56:51–65. - PubMed
-
- Naghavi M., Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. - DOI - PMC - PubMed
-
- National Cancer Institute: Childhood Cancers. [(accessed on 4 November 2017)]; Available online: https://www.cancer.gov/types/childhood-cancers.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous