Cellular and Molecular Aspects of the β-N-Methylamino-l-alanine (BMAA) Mode of Action within the Neurodegenerative Pathway: Facts and Controversy
- PMID: 29271898
- PMCID: PMC5793093
- DOI: 10.3390/toxins10010006
Cellular and Molecular Aspects of the β-N-Methylamino-l-alanine (BMAA) Mode of Action within the Neurodegenerative Pathway: Facts and Controversy
Abstract
The implication of the cyanotoxin β-N-methylamino-l-alanine (BMAA) in long-lasting neurodegenerative disorders is still a matter of controversy. It has been alleged that chronic ingestion of BMAA through the food chain could be a causative agent of amyotrophic lateral sclerosis (ALS) and several related pathologies including Parkinson syndrome. Both in vitro and in vivo studies of the BMAA mode of action have focused on different molecular targets, demonstrating its toxicity to neuronal cells, especially motoneurons, and linking it to human neurodegenerative diseases. Historically, the hypothesis of BMAA-induced excitotoxicity following the stimulation of glutamate receptors has been established. However, in this paradigm, most studies have shown acute, rather than chronic effects of BMAA. More recently, the interaction of this toxin with neuromelanin, a pigment present in the nervous system, has opened a new research perspective. The issues raised by this toxin are related to its kinetics of action, and its possible incorporation into cellular proteins. It appears that BMAA neurotoxic activity involves different targets through several mechanisms known to favour the development of neurodegenerative processes.
Keywords: BMAA; excitotoxicity; glutamate receptor; intracellular calcium; neurodegenerative disorders; neuromelanin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
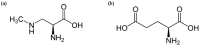



Similar articles
-
beta-N-methylamino-l-alanine induces oxidative stress and glutamate release through action on system Xc(-).Exp Neurol. 2009 Jun;217(2):429-33. doi: 10.1016/j.expneurol.2009.04.002. Epub 2009 Apr 15. Exp Neurol. 2009. PMID: 19374900
-
Retention of the cyanobacterial neurotoxin beta-N-methylamino-l-alanine in melanin and neuromelanin-containing cells--a possible link between Parkinson-dementia complex and pigmentary retinopathy.Pigment Cell Melanoma Res. 2009 Feb;22(1):120-30. doi: 10.1111/j.1755-148X.2008.00508.x. Pigment Cell Melanoma Res. 2009. PMID: 19154235
-
Investigation of the interaction of β-methylamino-L-alanine with eukaryotic and prokaryotic proteins.Amino Acids. 2018 Apr;50(3-4):397-407. doi: 10.1007/s00726-017-2525-z. Epub 2017 Dec 12. Amino Acids. 2018. PMID: 29235019
-
Neuropathological Mechanisms of β-N-Methylamino-L-Alanine (BMAA) with a Focus on Iron Overload and Ferroptosis.Neurotox Res. 2022 Apr;40(2):614-635. doi: 10.1007/s12640-021-00455-6. Epub 2022 Jan 13. Neurotox Res. 2022. PMID: 35023054 Review.
-
Is there a role for naturally occurring cyanobacterial toxins in neurodegeneration? The beta-N-methylamino-L-alanine (BMAA) paradigm.Neurochem Int. 2007 Jun;50(7-8):998-1003. doi: 10.1016/j.neuint.2006.12.011. Epub 2007 Jan 14. Neurochem Int. 2007. PMID: 17296249 Review.
Cited by
-
β-N-Methylamino-L-Alanine (BMAA) Modulates the Sympathetic Regulation and Homeostasis of Polyamines.Toxins (Basel). 2023 Feb 9;15(2):141. doi: 10.3390/toxins15020141. Toxins (Basel). 2023. PMID: 36828455 Free PMC article.
-
Effect of Microcystin-LR, Nodularin, Anatoxin-a, β-N-Methylamino-L-Alanine and Domoic Acid on Antioxidant Properties of Glutathione.Life (Basel). 2022 Jan 31;12(2):227. doi: 10.3390/life12020227. Life (Basel). 2022. PMID: 35207513 Free PMC article.
-
Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Dangerous Liaisons.Int J Mol Sci. 2021 Aug 13;22(16):8726. doi: 10.3390/ijms22168726. Int J Mol Sci. 2021. PMID: 34445429 Free PMC article. Review.
-
Microbial BMAA elicits mitochondrial dysfunction, innate immunity activation, and Alzheimer's disease features in cortical neurons.J Neuroinflammation. 2020 Nov 5;17(1):332. doi: 10.1186/s12974-020-02004-y. J Neuroinflammation. 2020. PMID: 33153477 Free PMC article.
-
Interaction of Neuromelanin with Xenobiotics and Consequences for Neurodegeneration; Promising Experimental Models.Antioxidants (Basel). 2021 May 21;10(6):824. doi: 10.3390/antiox10060824. Antioxidants (Basel). 2021. PMID: 34064062 Free PMC article. Review.
References
-
- Cox P.A., Banack S.A., Murch S.J., Rasmussen U., Tien G., Bidigare R.R., Metcalf J.S., Morrison L.F., Codd G.A., Bergman B. Diverse taxa of cyanobacteria produce β-N-methylamino-l-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA. 2005;102:5074–5078. doi: 10.1073/pnas.0501526102. - DOI - PMC - PubMed
-
- Réveillon D., Séchet V., Hess P., Amzil Z. Production of BMAA and DAB by diatoms (Phaeodactylum tricornutum, Chaetoceros sp., Chaetoceros calcitrans and Thalassiosira pseudonana) and bacteria isolated from a diatom culture. Harmful Algae. 2016;58:45–50. doi: 10.1016/j.hal.2016.07.008. - DOI - PubMed
-
- Lage S., Costa P.R., Moita T., Eriksson J., Rasmussen U., Rydberg S.J. BMAA in shellfish from two Portuguese transitional water bodies suggests the marine dinoflagellate Gymnodinium catenatum as a potential BMAA source. Aquat. Toxicol. 2014;152:131–138. doi: 10.1016/j.aquatox.2014.03.029. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

