Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling
- PMID: 29271910
- PMCID: PMC5795977
- DOI: 10.3390/ijms19010026
Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling
Abstract
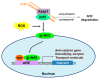
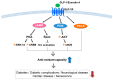
Oxidative cellular damage caused by free radicals is known to contribute to the pathogenesis of various diseases such as cancer, diabetes, and neurodegenerative diseases, as well as to aging. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) and Kelch-like ECH-associated protein1 (Keap1) signaling pathways play an important role in preventing stresses including oxidative and inflammatory stresses. Nrf2 is a master regulator of cellular stress responses, induces the expression of antioxidant and detoxification enzymes, and protects against oxidative stress-induced cell damage. Glucagon-like peptide-1 (GLP-1) is an incretin hormone, which was originally found to increase insulin synthesis and secretion. It is now widely accepted that GLP-1 has multiple functions beyond glucose control in various tissues and organs including brain, kidney, and heart. GLP-1 and GLP-1 receptor agonists are known to be effective in many chronic diseases, including diabetes, via antioxidative mechanisms. In this review, we summarize the current knowledge regarding the role of GLP-1 in the protection against oxidative damage and the activation of the Nrf2 signaling pathway.
Keywords: Nrf2 signaling; diabetes; glucagon-like peptide-1; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tebay L.E., Robertson H., Durant S.T., Vitale S.R., Penning T.M., Dinkova-Kostova A.T., Hayes J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015;88:108–146. doi: 10.1016/j.freeradbiomed.2015.06.021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

