Chaperones and the Proteasome System: Regulating the Construction and Demolition of Striated Muscle
- PMID: 29271938
- PMCID: PMC5795982
- DOI: 10.3390/ijms19010032
Chaperones and the Proteasome System: Regulating the Construction and Demolition of Striated Muscle
Abstract
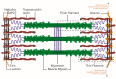
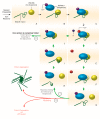
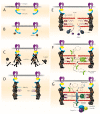
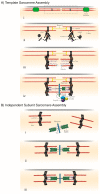
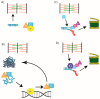
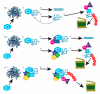
Protein folding factors (chaperones) are required for many diverse cellular functions. In striated muscle, chaperones are required for contractile protein function, as well as the larger scale assembly of the basic unit of muscle, the sarcomere. The sarcomere is complex and composed of hundreds of proteins and the number of proteins and processes recognized to be regulated by chaperones has increased dramatically over the past decade. Research in the past ten years has begun to discover and characterize the chaperones involved in the assembly of the sarcomere at a rapid rate. Because of the dynamic nature of muscle, wear and tear damage is inevitable. Several systems, including chaperones and the ubiquitin proteasome system (UPS), have evolved to regulate protein turnover. Much of our knowledge of muscle development focuses on the formation of the sarcomere but recent work has begun to elucidate the requirement and role of chaperones and the UPS in sarcomere maintenance and disease. This review will cover the roles of chaperones in sarcomere assembly, the importance of chaperone homeostasis and the cooperation of chaperones and the UPS in sarcomere integrity and disease.
Keywords: HSP; homeostasis; misfolded protein; molecular chaperone; protein complex assembly; protein degradation; sarcomere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bianchi T., Gelosa L. On the preventive detection of pathogenic staphylococci in the rhinopharynx of employees in the food industry in the province of Milan during the 5-year period of 1967–1971. Ann. Sclavo. 1973;15:83–98. - PubMed
-
- Kony D.B., Hunenberger P.H., van Gunsteren W.F. Molecular dynamics simulations of the native and partially folded states of ubiquitin: Influence of methanol cosolvent, pH, and temperature on the protein structure and dynamics. Protein Sci. 2007;16:1101–1118. doi: 10.1110/ps.062323407. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

